Figure 1.
Regulation of DLC3 membrane association by a novel polybasic region
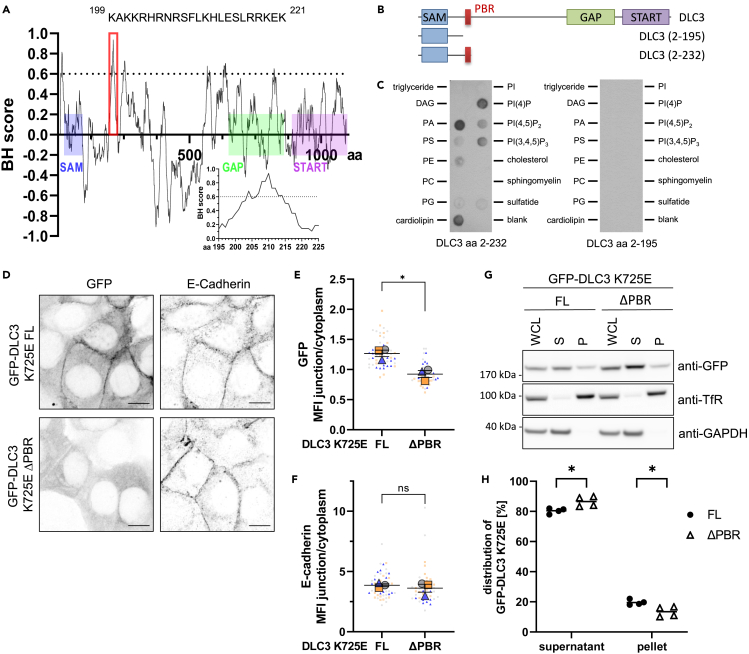
(A) BH plot of basic and hydrophobic residues in DLC3 using the scale developed by Brzseska et al. The relative localization of the SAM, GAP and START domains are schematically annotated on the profile. The red box marks the identified polybasic region (PBR) spanning amino acids (aa) 199–221 with the sequence given. The blot of this region is magnified in the insert.
(B) Line diagram showing the domain organization of full-length DLC3 and fragments used for the lipid overlay assay in (C), with the PBR marked in red.
(C) Recombinant GST-tagged N-terminal DLC3 fragments containing the PBR (left) or lacking the PBR (right) were incubated with lipid strips. Bound protein was detected by immunoblotting with anti-GST antibody, followed by HRP-coupled secondary antibody. DAG = diacylglycerol, PA = phosphatidic acid, PS = phosphatidylserine, PE = phosphatidylethanolamine, PC = phosphatidylcholine, PG = phosphatidylglycerol, PI = phosphatidylinositol, sulfatide = 3-sulfogalactosylceramide.
(D) Localization of GFP-DLC3 K725E full-length (FL) and ΔPBR in MCF7 cells inducibly expressing GFP-DLC3. E-cadherin-specific immunostainings. Images are maximum intensity projections of several confocal sections. Scale bars: 10 μm.
(E and F) Analysis of images from (D). Graph shows the mean fluorescence intensity (MFI ±SEM) of the signal at cell junctions versus the cytoplasmic signal for GFP (E) or E-cadherin (F) (n = 3; N = 50, 43 cells; t test: p = 0.0123 (E), p = 0.5194, ns = not significant (F)).
(G) Biochemical fractionation of MCF7 cells stably expressing GFP-DLC3 K725E or K725E ΔPBR into soluble supernatant and membrane-containing pellet fractions. Fractions were analyzed by immunoblotting with the indicated antibodies followed by HRP-coupled secondary antibody.
(H) Shown is the distribution of GFP signal in the immunoblotted fractions analyzed by Fiji, normalized to GAPDH (supernatant fraction) or transferrin receptor (pellet fraction) (line shows mean of 4 independent experiments; two-way ANOVA with Sidak’s multiple comparison test: p = 0.0147).