Figure 2.
DLC3 PBR phosphorylation impairs membrane interaction in vitro
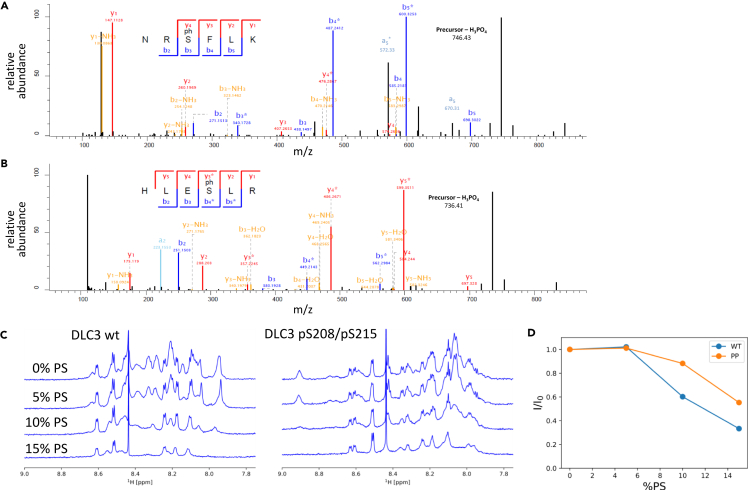
(A and B) Fragmentation mass spectra of the phosphopeptides NRpSFLK (A) and HLEpSLR (B) corresponding to amino acids 206–211 and 212–217 in DLC3, respectively, obtained from immunoprecipitated FLAG-tagged DLC3.
(C) Superposition of the NMR spectra of 0.1 mM peptides encompassing DLC3 aa 199–221 wt (left) or phosphorylated on serines 208 and 215 (right) measured in the presence of unilamellar vesicles containing variable amounts of the negatively charged POPS. From top to bottom: 100% POPC, 5% POPS/95% POPC, 10% POPS/90% POPC, 15% POPS/85% POPC, total lipid concentration 2 mM. The intensity of the NMR signals progressively decrease due to the increasing interaction of the PBR peptide with the negatively charged vesicles.
(D) The dependence of the total integral intensity (I) of the NH signals on the POPS percentage in the vesicles (%PS) presented as a ratio to the integral intensity at 0% POPS (I0) that quantifies the signal reduction. The signal intensities of the doubly phosphorylated peptide decrease less than the unmodified peptide, indicating reduced interaction with the membrane upon phosphorylation.