Figure 4.
A PBR-dependent role for DLC3 in the regulation of cell division
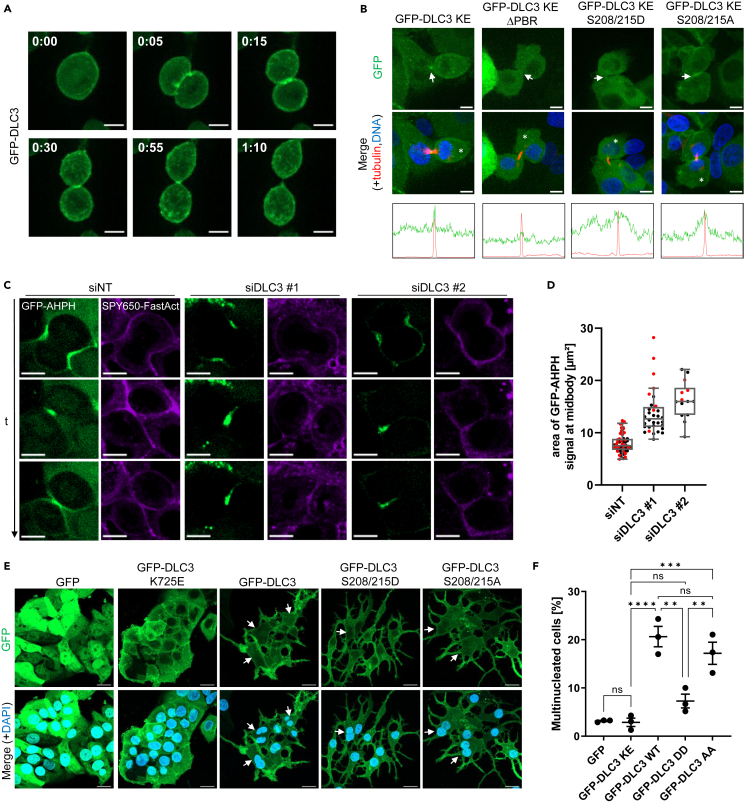
(A) Expression of GFP-DLC3 in stable MCF7 cells was induced for 24 h with doxycycline and cells were analyzed by live-cell imaging. Time stamp: h:mm, scale bars: 10 μm.
(B) Expression of indicated GFP-tagged DLC3 constructs (green) in stable MCF7 cells was induced for 24 h with doxycycline and cells were analyzed by live-cell imaging. Midbodies, indicated by arrows, were identified using SPY555-tubulin staining (red). Nuclei were counterstained with SPY650-DNA. Line plots show mean fluorescence signal along the perimeter of cells marked with asterisks. Scale bars: 10 μm.
(C) MCF7 cells stably expressing the Rho-GTP biosensor GFP-AHPH (green) were transfected with the indicated siRNAs. After 72 h, cells were stained with SPY650-FastAct (magenta) and analyzed by live-cell imaging. Representative maximum intensity projections of selected time frames from live-cell imaging movies are shown. Scale bars: 10 μm.
(D) The area of GFP-AHPH sensor signal in cells from (C) at the midbody area was quantified with Fiji (n = 2; N = 57, 32, 14) and normalized to control siRNA. Graph shows individual sample points and means in a boxplot with Tukey whiskers.
(E) Expression of indicated GFP-tagged DLC3 constructs in stable MCF7 cells was induced for 72 h with doxycycline. Cells were fixed, nuclei were counterstained with DAPI and samples analyzed by fluorescence microscopy. Multinucleated cells are marked with an arrow. Scale bars: 20 μm.
(F) The percentage of multinucleated cells was determined manually. Graph shows the means (±SEM) of three independent experiments (n = 3; N = 318, 324, 189, 173, 183). KE: GAP-inactive K725E mutation, WT: wild-type, DD: phosphomimetic S208/215D mutations, AA: phosphodeficient S208/215A mutations. One-way ANOVA with Tukey’s post-test: GFP vs. KE p = 0.99994; KE vs. WT p = 0.00009; KE vs. DD p = 0.34386; KE vs. AA p = 0.00058; WT vs. DD p = 0.00100; WT vs. AA p = 0.55891; DD vs. AA p = 0.00875.