Fig. 5. Model of the host responses to M.tb in the lung of PLWH compared to control.
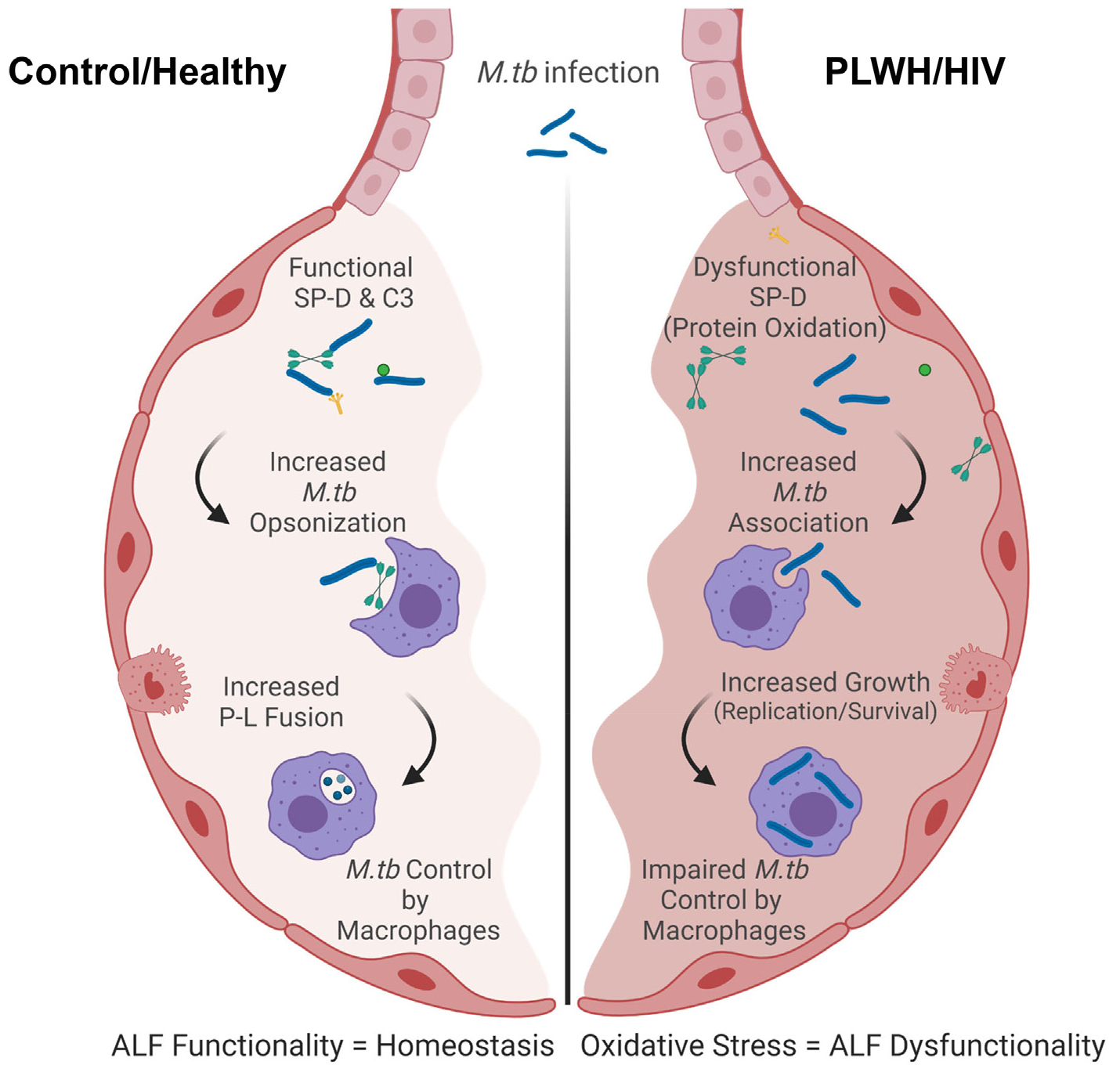
Local oxidative stress is detected in the lung of PLWH. This oxidative stress may drive the dysfunction of soluble immune components and inflammation in the lungs marked by decreased levels of Th1/Th2/Th17 cytokines. Lower levels of complement and SP-D are also detected in PLWH leading to their lower capacity to opsonize and/or modify the M.tb bacterial cell surface prior contacting host cells. This drives better recognition of HIV-ALF exposed M.tb by macrophages. Phagocytosed HIV-ALF exposed M.tb bacilli grow faster within macrophages by further impairing P-L fusion and replicating faster within phagosomes. In vitro, this is related to SP-D function since addition of excess of SP-D to HIV-ALF restores the capacity of macrophages to control HIV-ALF-exposed M.tb; thus, SP-D plays an important role in maintaining lung homeostasis and innate immune activity against lung respiratory infections. Created in BioRender.com.
