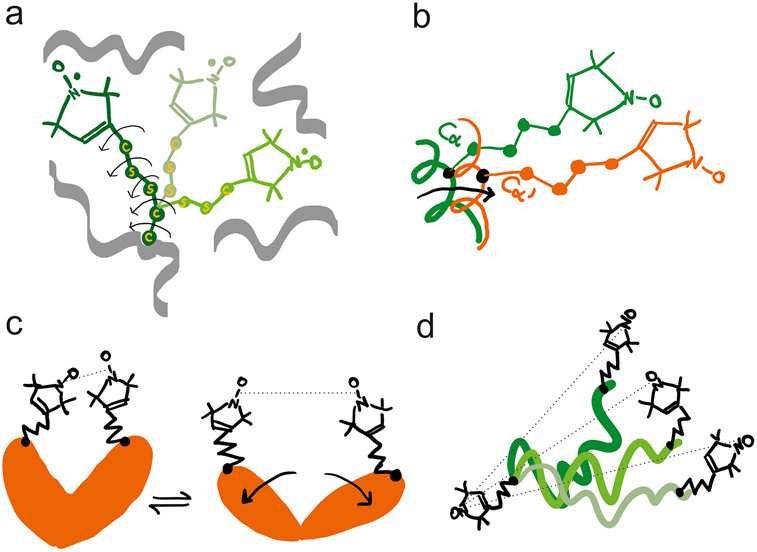
Figure 5.
Schematic representation of the most common determinants of the width of the MTSL-derived distance distributions in PELDOR/DEER. (a) The five potentially rotatable bonds enable distinct rotamers to be populated at a specific site, based on the steric hindrance imposed by neighboring side chains and backbone atoms. Three rotamers are shown with arrows highlighting the rotatable bonds in one of them. (b) Small translational or rotational motion of the backbone to which the rotamers are attached can also induce broadening or appearance of shoulders in the distance distribution toward another spin-labeled site. (c) The protein adopts two distinct conformations (for example with and without a ligand bound) which can be monitored by PELDOR/DEER. Equilibria between two conformations can also be identified by the appearance of two peaks in the distance distribution. (d) If MTSL is attached to intrinsically disordered proteins or to a dynamic region of a protein, a broad distribution of distances is expected in frozen state. Such disorder is correlated with the large-amplitude motions of the backbone to which MTSL is attached.