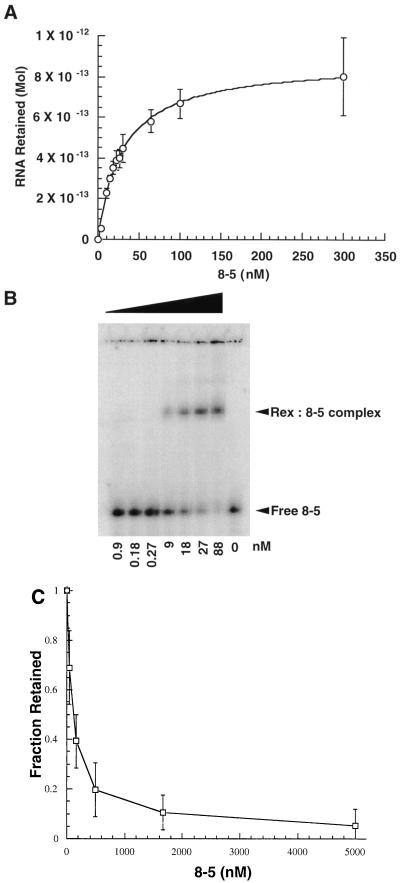
FIG. 4.
Characterizing interactions between aptamer 8-5 and the Rex protein. (A) RNA titration of the high-affinity aptamer 8-5. The Kapp of the high-affinity aptamer (30 nM) was determined by titrating increasing amounts of RNA against a constant amount of protein. Samples were filtered through modified nitrocellulose, and counts retained were quantitated with a PhosphorImager. (B) Gel mobility shift assay of the high-affinity aptamer 8-5. An increasing amount of Rex fusion protein was titrated against a constant amount of body-labeled aptamer (10 ng). Complexes were separated by electrophoresis in an 8% native acrylamide gel in 1× TBE at room temperature. The amount of complex formed as a function of protein concentration was determined with a PhosphorImager. (C) Competition assay of the high-affinity aptamer 8-5 with stem IID of the XRE. Competition for binding to stem IID of the XRE was measured by incubating increasing amounts of aptamer 8-5 with a constant amount of body-labeled stem IID (0.5 μM) in the presence of the Rex protein. As the concentration of unlabeled aptamer increased, a corresponding decrease in the amount of stem IID retained following filtration was observed. A least squares fit of the data indicated that 50% inhibition of complex formation occurred at an RNA concentration of 100 nM. In contrast, no consistent diminution in binding was observed with a nonspecific RNA competitor, tRNA.