Abstract
Maturation and release of human immunodeficiency virus type 1 (HIV-1) is targeted at the pseudopod of infected mononuclear cells. However, the intracellular mechanism or targeting signals leading to this polarized viral maturation are yet to be identified. We have recently demonstrated the presence of a functional YXXL motif for specific targeting of HIV-1 virions to the basolateral membrane surface in polarized epithelial Madin-Darby canine kidney cells (MDCK). Site-directed mutagenesis was used to demonstrate that the membrane-proximal tyrosine in the intracytoplasmic tail of the HIV-1 transmembrane glycoprotein (gp41) is an essential component of this signal. In the present study, immunolocalization of viral budding allowed us to establish that this tyrosine-based signal is involved in determining the exact site of viral release at the surface of infected mononuclear cells. Substitution of the critical tyrosine residue was also shown to increase the amount of envelope glycoprotein at the cell surface, supporting previous suggestions that the tyrosine-based motif can promote endocytosis. Although alteration of the dual polarization-endocytosis motif did not affect the infectivity of cell-free virus, it could play a key role in cell-to-cell viral transmission. Accordingly, chronically infected lymphocytes showed a reduced ability to transmit the mutant virus to a cocultivated cell line. Overall, our data indicate that the YXXL targeting motif of HIV is active in various cell types and could play an important role in viral propagation; this may constitute an alternative target for HIV therapeutics and vaccine development.
The last step in the viral multiplication cycle of enveloped viruses is the maturation and release of viral particles together with acquisition of the lipid envelope. In most retroviruses, including human immunodeficiency virus type 1 (HIV-1), this is accomplished by budding at the plasma membrane, where the viral envelope glycoproteins are incorporated into the released virions. Retroviruses are peculiar among enveloped viruses in that viral glycoproteins are not required for the actual budding process (7, 16, 41). In the absence of these proteins, the viral capsids are assembled and released with their lipid envelope devoid of viral glycoproteins; as a result, the virions produced are noninfectious.
It is now well established that the plasma membrane surface of eucaryotic cells presents distinct protein and lipid compositions in specific membrane domains (4, 18, 42, 50). Although most studies have been performed with epithelial cells, in which well-defined apical and basolateral surfaces can be distinguished, the same phenomenon also seems to exist in other cell types. Lymphocytes are one such example of cells exhibiting a definite form of polarization at their membrane surface. This has been best demonstrated on activated lymphocytes, which developed cytoplasmic projections called uropodes (2, 56, 57, 58). It was also shown that specific cytokines are able to induce this polarization phenotype (8).
In accordance with the differentiated nature of the eucaryotic plasma membrane, viral budding is often seen as “polarized,” being targeted to specific regions of the cell surface. This most often results from the transport of viral envelope glycoproteins to the corresponding membrane regions. This phenomenon has been studied mostly with epithelial cells, showing that different viruses will preferentially or exclusively bud from either the apical or basolateral membrane domain (44, 45, 53). There is much less information about polarized viral release in other cell types. It has been reported that viral budding can occur from either axonal extensions or the cell body in infected neuronal cells, with the site of budding depending on the virus examined (6, 9, 10, 43, 55).
In the last few years, evidence of a polarized release of HIV-1 in epithelial cells has been accumulating. It has been shown that this release is generally restricted to the basolateral surface. This is clearly due to targeting of the envelope glycoprotein since, in its absence, viral release occurs from both the apical and basolateral poles of the cells (13, 19, 27, 34). Interestingly, budding of HIV virions was also seen to be polarized at the surface of infected lymphocytes (12, 36, 38, 39, 48). Viral release is observed mostly at one pole, generally corresponding to contact sites between lymphocytes or with a solid support (35, 36). Both types of contact may generate a cell surface analogous to the basolateral domain of epithelial cells. Supporting this idea, it has long been known that vesicular stomatitis virus destined for the basolateral surface of epithelial cells is released through these contact sites when isolated epithelial cells are allowed to reattach to a solid support or interact with each other in suspension (42, 45). It has also been suggested that cytoskeletal elements located underneath the cell surface may play an important role in the differentiation of distinct membrane domains for epithelial cells, as well as in the reorganization of cell morphology for lymphocytes (2, 32, 54). A specific distribution of cytoskeletal elements underlying sites of HIV budding was shown and supports the idea that cytoskeletal elements are also involved in defining viral assembly sites (37).
Although their exact nature remains controversial, it is now generally believed that specific targeting signals are present on proteins destined for different membrane domains. Basolateral signals seem to consist of either dileucine motifs (26) or, more commonly, tyrosine-based motifs in a general Y-X-X-(aliphatic or aromatic) consensus (18, 20, 28, 31, 51). Such tyrosine-based motifs are also often associated with endocytosis signals, and there can be an overlap between these two classes of motifs (5, 11, 47, 49). It has been reported that replacement of a tyrosine, analogous to the substitutions shown to abolish HIV polarized budding in epithelial cells, results in decreased endocytosis and accumulation of envelope glycoproteins at the cell surface of lymphocytes infected with either HIV-1 or simian immunodeficiency virus (SIV) (24, 47, 49). Some evidence was also presented for a uniform cell surface glycoprotein distribution of a similar SIV mutant, in contrast to the wild-type envelope, which appears to be restricted mostly to one pole of lymphocytes (11, 24, 49).
In the present study, mutant HIV strains altered in their basolateral polarization signal were examined in infected mononuclear cells to determine if the tyrosine-based polarization signal of HIV is also active in such cell types and could play a role in viral pathogenesis.
MATERIALS AND METHODS
Proviral constructs.
Site-directed mutagenesis of envelope expression vectors has been described previously (31). A KpnI-KpnI fragment (nucleotides 3701 to 5893) from the wild-type HIV provirus HXBc2 (14, 40) was introduced in the mutated envelope expression vectors to reestablish the original sequences essential for Tat expression (15). Proviral constructs were derived by subcloning a SalI-BamHI fragment (nucleotides 5331 to 8017) from these different mutated hybrid envelope fragments into an HXBc2 proviral construct harboring a BglII-BglII deletion (nucleotides 7163 to 7628) in the region encoding the envelope glycoprotein. This strategy facilitated the screening of proviruses reconstructed with the different mutated but full-length glycoprotein-encoding fragments. All mutations were confirmed by DNA sequencing.
Cells.
The Jurkat CD4+ human lymphoid cell line was maintained in RPMI 1640 medium containing 10% fetal calf serum and 1% antibiotics (3). The simian virus 40-transformed African green monkey kidney COS-7 and the HeLa-CD4-LTR–β-gal cell lines used in this study were maintained in Dulbecco’s modified Eagle’s medium supplemented with 8% fetal calf serum and 1% antibiotics. Human peripheral blood mononuclear cells (PBMCs) from a normal donor were purified and activated as previously described (29).
Transfection of Jurkat lymphocytes.
Human Jurkat lymphocytic cells were transfected with the different proviral DNA constructs (15 μg of proviral DNA per 107 cells) by using a DEAE-dextran technique essentially as described previously (60). The number of surviving cells was determined every 3 days by using trypan blue exclusion as a criteria for viability. The cells were then centrifuged, resuspended in fresh medium, and diluted to a concentration of 500,000 cells/ml for further growth. After maximum cell death and cytotoxicity induced by HIV during viral multiplication, the remaining cells were amplified as a total population and used for subsequent experiments. In some experiments, the chronically infected Jurkat cells were activated overnight with 12-phorbol-13-myristate acetate (PMA; Sigma) at a final concentration of 160 nM to upregulate HIV-1 gene expression (46).
Infection of human PBMCs.
Infectious virus was recovered from COS cells transfected with either wild-type or mutant proviral DNAs, and its titer was determined with the HeLa–CD4–LTR–β-gal indicator cell line (23). Activated PBMCs were then infected at a multiplicity of infection of 1. Development of the infection was monitored as described for Jurkat cells by measuring both cell viability and viral reverse transcriptase activity (25).
FACS analysis.
The presence of viral envelope glycoprotein at the cell surface was measured with a fluorescence-activated cell sorter (FACS). Cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) before being processed. Briefly, the cells were incubated in a 1/200 dilution of human anti-HIV antiserum 162 and, following adsorption for 1 h on ice, washed three times with PBS and reincubated with a 1/100 dilution of fluorescein-conjugated goat anti-human antibody (Boehringer Mannheim). Following incubation and three washes in PBS, the cells were analyzed with a FACStar cytofluorometer (Beckton Dickinson).
Immunofluorescence analysis.
Immunological detection of p24 capsid protein was performed with cells dried on 15-well slides. The cells were fixed and permeabilized with a 50:50 mixture of cold acetone and methanol, preincubated for 30 min at 25°C in PBS containing 2% nonfat milk, and then incubated for 2 h with a monoclonal mouse anti-p24 capsid protein without dilution. The cells were then washed and reincubated for 30 min with a 1:50 dilution of fluorescein-conjugated goat anti-mouse antibody before being given their final washes. To determine the percentage of polarization, the cells were examined by fluorescence microscopy with a Axioskop microscope (Zeiss), using adequate filters for visualization of green fluorescence (fluorescein with a 530-nm wavelength). Confocal laser scanning microscopy was performed with an LSM 410 microscope (Zeiss) equipped with a Pln-APOCHROMAT 63× oil immersion objective and an Ar/Kr laser. The fluorescein isothiocyanate images were obtained by scanning the cells with the 488-nm laser and filtering the emission with a 515- to 540-nm band-pass filter. For each cell studied, an image of the additive signal through its whole thickness was first digitized and the confocal serial sections were then scanned.
Efficiency of cell-to-cell viral transmission.
Chronically infected nonadherent Jurkat cells, producing either wild-type or Y712S virus, were added at a very low concentration (3,000 cells) to the indicator cell line HeLa-CD4-LTR–β-gal cells (300,000 cells) in six-wells plates in duplicate. After intercellular contact for different times (1, 4, or 16 h), the indicator epithelial cells were washed and cultivated for 48 h before the cells were stained for β-galactosidase expression as previously described (23); positive (blue) cells were then counted under the microscope at ×100 magnification. Infectivity of cell-free virus was tested by infection of HeLa-CD4-LTR–β-gal cells with the equivalent of 300,000 cpm of reverse transcriptase activity determined on 50-μl aliquots (25); staining for β-galactosidase expression was performed 48 h later.
RESULTS
Cell surface expression of mutant glycoproteins.
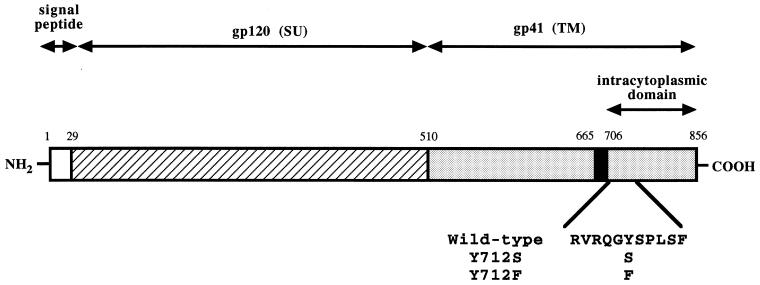
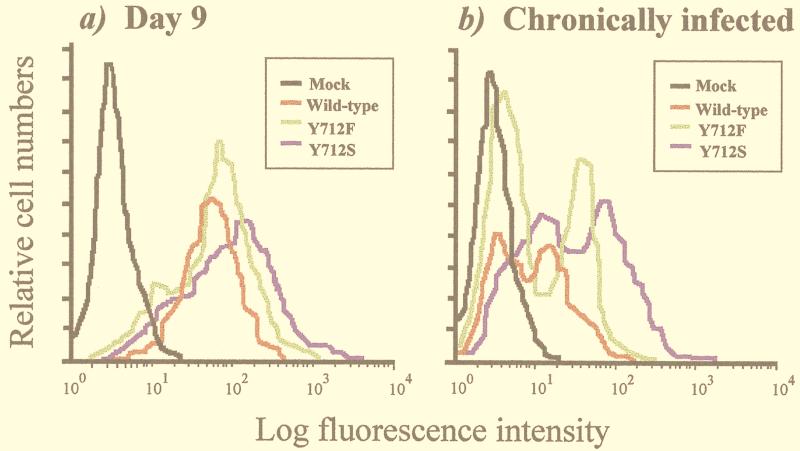
Chronically infected Jurkat CD4+ lymphocytes were established by transfection of plasmids containing either wild-type or mutated HIV-1 proviral DNA to assess the functional properties of mutant envelope glycoproteins in lymphocytes. It has been previously established that virus mutants can replicate efficiently in such transfected cells. Kinetics of cell killing, development of viral reverse transcriptase activity, peak reverse transcriptase levels, and reverse transcriptase levels following establishment of chronically infected cells were all identical when wild-type and mutant viruses were compared (reference 28 and unpublished data). In these mutants (Fig. 1), the intracytoplasmic membrane-proximal tyrosine critical in the basolateral targeting signal (tyrosine 712) was replaced by a serine residue (Y712S), a nonconservative substitution that removes the aromatic ring while keeping a hydroxyl side chain. Alternatively, the same tyrosine was replaced by an alanine (Y712A; nonconservative substitution) or a phenylalanine (Y712F; the aromatic ring is conserved). The level of envelope glycoprotein at the cell surface was first examined by flow cytometric analysis (FACS). The analysis was performed on cells taken at 9 days posttransfection, a time point slightly before the maximal virus titer was achieved and corresponding to the peak in cell death and syncytium formation (28); at this point, essentially all the cells were infected and strongly positive by the p24 immunofluorescence assay (data not shown). Substitutions of the membrane-proximal tyrosine 712 residue result in an increased level of cell surface glycoprotein (Fig. 2a); the mean fluorescence intensity was about threefold higher in cells expressing the Y712S (or Y712A) mutant than in cells expressing the wild type and was 50% higher in cells expressing the Y712F mutant than in cells expressing the wild type. In parallel, the same analysis was performed on chronically infected lymphocytes (21 days posttransfection) induced with PMA; this treatment favors the upregulation of HIV-1 gene expression (46). Under these conditions, two different cell populations were observed by FACS analysis, and the mean fluorescence intensity in the population of strongly positive cells was still two- to threefold lower than that observed at the peak of viral production (Fig. 2b). Nevertheless, the relative levels of surface envelope glycoproteins observed with the different mutants were similar when such chronically infected cell populations were compared to the cells at the peak of viral production. Again, replacement of tyrosine 712 with a serine (or alanine) and, to a lesser extent, conservative replacement with a phenylalanine increase the level of envelope glycoprotein at the cell surface. It therefore appears that the amount of cell surface envelope glycoprotein is normally downmodulated by the amino acid motif encompassing the membrane-proximal tyrosine residue. This phenomenon is observed in infected cells and is still observed at the time of chronic infection.
FIG. 1.
Schematic representation of the HIV envelope glycoprotein. A schematic view of the glycoprotein primary structure (856 amino acids) is presented. The amino acid sequence of the intracytoplasmic juxtamembrane region is presented underneath, as well as the nature of the mutations used in the present study.
FIG. 2.
Cell surface expression of the HIV envelope glycoprotein. Transfected Jurkat cells were subjected to FACS analysis with an anti-HIV antiserum on unfixed cells. The analysis was performed on day 9 posttransfection (a) as well as on chronically infected Jurkat cells induced with PMA soon after chronic infection was established (b). For both panels, results are presented for the mock infection (noninfected) and infection with wild-type, mutant Y712S (identical results were obtained with Y712A), and mutant Y712F viruses.
Polarization of viral budding.
The site of viral budding at the cell surface of infected Jurkat lymphocytes was examined to determine if mutation of the basolateral polarization signal could also affect the localization of viral budding in these cells. Permeabilization of the cells prior to the immunodetection procedure allowed us to use an antibody directed against the major capsid protein p24 to localize the region of the cell surface where assembly and budding of the capsid occur. A preferential viral budding through one pole of the cell was easily demonstrated and was best visualized by confocal microscopy examination of serial sections of cells infected with wild-type virus at the time of peak viral production (Fig. 3a). Since these experiments were performed on fixed and permeabilized cells for the detection of a nonmembrane protein, the phenomenon observed could not be due to antibody “capping” but must be the consequence of an actual transport of the protein to this specific region underneath the cell membrane surface. Essentially identical results were observed when chronically infected cells were examined, except that only a percentage of the cells had a sufficient expression level for immunofluorescence detection (data not shown).
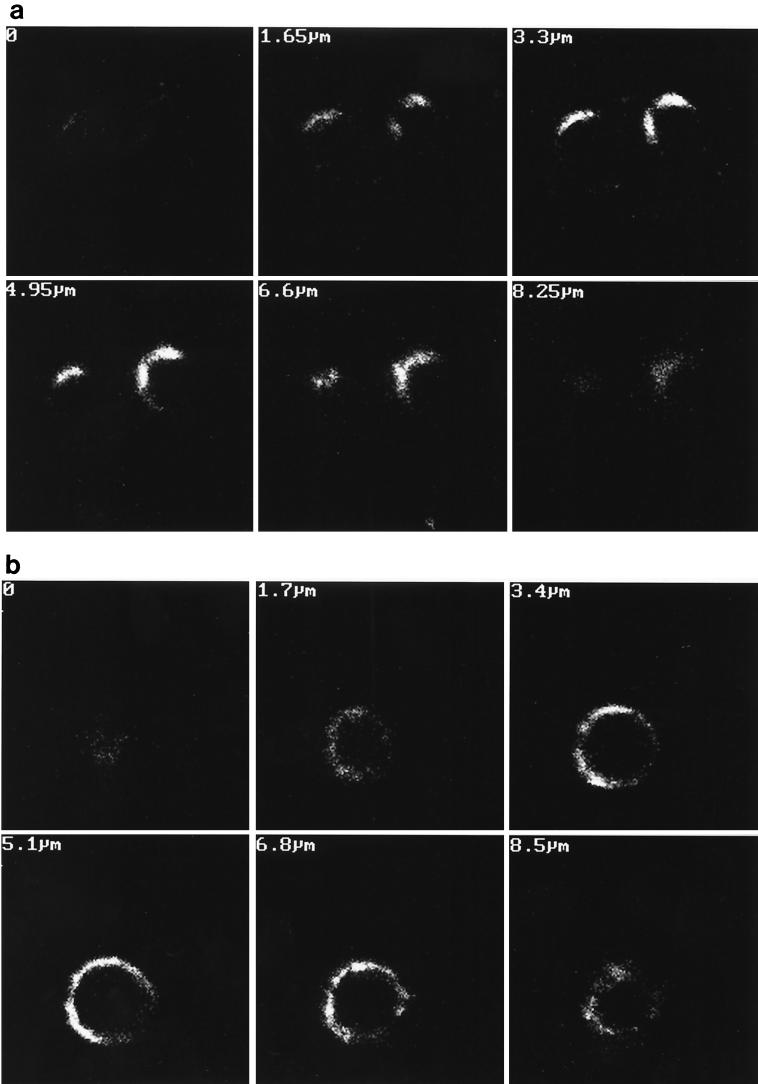
FIG. 3.
Polarized localization of p24. Transfected Jurkat cells at the peak of viral replication (9 days posttransfection) were analyzed by confocal microscopy with a monoclonal anti-p24 antibody as described in Materials and Methods. Cells transfected with wild-type proviral DNA exhibit polarized localization of the p24 protein (a), while cells transfected with mutant Y712S DNA showed a lack of polarization (b). Numbers in top left corners indicate the depth of each serial section. Magnification, ×360.
When the different mutants were examined, it was quite clear that the membrane-proximal tyrosine was essential for the polarization phenotype, as previously observed in epithelial cells (27, 28). The distribution of fluorescence appeared uniform, without any apparent patches or preferential pole of release at the surface of lymphocytes infected with viruses whose membrane-proximal tyrosine was substituted (Fig. 3b).
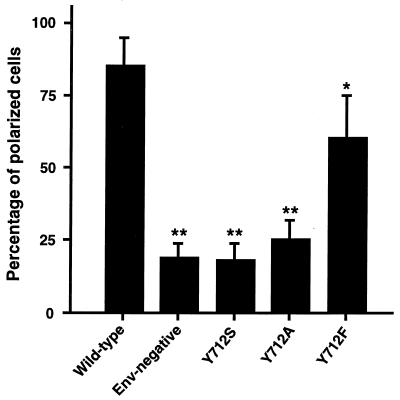
To quantitate the data, cells processed for immunofluorescence were examined by two different investigators who were unaware of the nature of the different samples. One hundred cells were counted for each sample, and each cell was scored positive or negative for the polarization phenotype (Fig. 4); cells were considered positive when the signal was restricted to less than half of the cell periphery. These data confirmed that tyrosine 712 is critical for the polarization phenotype of viral budding in lymphocytes since only 20 to 25% of the cells infected with the mutant were scored as exhibiting an apparent polarized p24 distribution while more than 85% of cells infected with the wild type exhibited this phenotype. A conservative replacement of tyrosine 712 by phenylalanine had only a partial effect, with 55 to 60% of the cells showing some evidence of polarization of viral budding. The presence of an aromatic ring thus appears critical for the polarization signal, as also observed for polarized budding in epithelial cells (28) and for the downmodulation of the viral glycoprotein at the cell surface (Fig. 2). The budding of a mutant virus defective for envelope expression was also examined. This mutant proviral DNA was trans-complemented by cotransfection with a wild-type envelope expression vector; the enveloped virions transiently produced in COS cells were then used to infect Jurkat lymphocytes. Budding of the resulting nonenveloped virions produced 48 h later in these lymphocytes was nonpolarized, with the p24 protein being located essentially all around the cells (Fig. 4 and data not shown). This further supports our assumption that polarization of viral budding is a property associated exclusively with the envelope glycoprotein.
FIG. 4.
Percentage of Jurkat cells exhibiting polarized p24 localization. Transfected Jurkat cells were permeabilized and examined by immunofluorescence with the anti-p24 antibody. Polarization was scored as positive when clear restriction of p24 staining to less than one-half of cell periphery was observed at one pole of the cell. The wild type, envelope-negative, and different mutant viruses were examined; in each case, 100 cells were examined and two independent readings were taken by two different investigators who were unaware of the nature of the different samples. Mean results are presented with error bars indicating standard deviation. Statistical significance was established by Student’s t test; significant differences from wild type at P < 0.05 (∗) or P < 0.005 (∗∗) are indicated.
In addition to the Jurkat cell line, the polarization of viral budding in primary human PBMCs was briefly investigated. These cells were infected with either wild-type or Y712S mutant virus, and both viruses were found to replicate with similar kinetics in these cells. Cells were taken at the time of peak viral replication and examined by immunofluorescence for p24 distribution. Despite the somewhat heterogeneous nature of this cell population, about 60% of wild-type-infected cells that exhibited a detectable immunofluorescence signal showed a concentration of this signal to a distinct membrane region; this polarization was apparently lost in the Y712S mutant since only 15 to 20% of the cells exhibited such a concentrated fluorescence signal (data not shown).
Polarization of viral release in Jurkat cells was finally confirmed by electron microscopy, which showed that the detection of p24 in a specific region of the cell surface did, in fact, reflect a viral budding specifically restricted at these plasma membrane regions. Wild-type virus was released exclusively at one pole of the cells, while the Y712S mutant was found in the form of complete or budding virions on various plasma membrane regions essentially all around the cells (data not shown).
Cell-to-cell viral transmission.
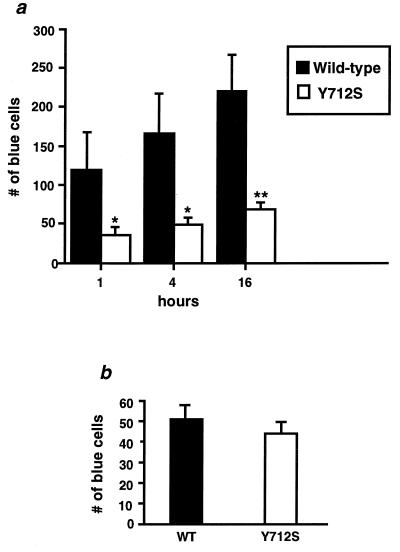
Having established that the tyrosine-based polarization signal is functional in human mononuclear cells, we examined the effect of this signal on viral multiplication. It was previously reported that the wild-type and mutant viruses affected in their polarization phenotype progressed at very similar rates in the transfected-cell population (28). However, in these experiments leading to the establishment of chronically infected cells, propagation via highly concentrated cell-free virus is probably predominant. A difference in the efficiency of early transmission by cell-to-cell contact was thus still a possibility and will be the most likely consequence of a change in the polarized-budding phenotype that could target viral budding at sites of intercellular contact. To assess more directly the effect of polarized release on viral transmission between cells, small numbers of Jurkat cells chronically infected with the wild-type or Y712S mutant virus were applied to the indicator cell line HeLa-CD4-LTR–β-gal, for 1, 4, or 16 h. After 48 h, the indicator cells were fixed and stained to investigate the efficiency of transmission between lymphocytes and epithelial cells. In this assay, transmission of viral infection was found to be three to four times more efficient from Jurkat cells chronically infected with the wild-type virus than from cells infected with the mutant (Fig. 5a). The difference observed could not be due to a reduction in viral infectivity per se. Chronically infected cells established with either wild-type or mutant viruses released identical amount of viruses, as established by their similar reverse transcriptase levels. These viruses were tested for their infectivity as cell-free viruses by using the same HeLa indicator cell line. As previously observed for transiently released viruses (28), the infectivity of mutant viruses released from chronically infected cells was found to be identical to that of wild-type virus in this assay (Fig. 5b).
FIG. 5.
Efficiency of cell-to-cell viral transmission. (a) Chronically infected Jurkat cells (3,000 cells) expressing either the wild type or the Y712S mutant were seeded in the presence of 300,000 HeLa-CD4-LTR–β-gal cells for 1, 4, or 16 h. The number of blue cells was counted 48 h after the beginning of the contact; the results are presented as the average of three independent experiments with error bars indicating standard deviation. (b) Viruses were recovered from the same chronically infected cell lines used in panel a. The same viral production, as measured by a reverse transcriptase assay, was observed in these cells, and the same volumes of virus inoculum were thus used to measure the infectivity of cell-free viruses on HeLa-CD4-LTR–β-gal cells. Results for the wild type (WT) and Y712S mutant are presented as the average of three independent experiments with error bars indicating standard deviation. Statistical significance was established by Student’s t test; significant differences from wild type at P < 0.02 (∗) or P < 0.005 (∗∗) are indicated.
DISCUSSION
Various evidence has previously led us to suspect that the targeting motif, previously identified as a basolateral signal for HIV-1 budding in epithelial cells, could play a role during viral maturation in human mononuclear cells. In epithelial cells, regions of contact with other cells or with a solid support are considered analogous to basolateral surfaces; viruses normally budding at the basolateral surface (for example, vesicular stomatitis virus) are specifically released at cell-to-cell or cell-substrate interfaces, while apically targeted viruses (for example, influenza virus) are released mostly through free cell surfaces. Series of observations have also shown a preferential budding of HIV-1 and human T-cell leukemia virus type 1 at sites where infected lymphocytes, or monocytes, are in contact with epithelial cells (1, 38, 39, 61). Additional studies revealed that this specific budding at one pole of infected cells is not restricted to intercellular contact sites but can also be found on individual infected monocytes (37).
In the present study, it has been clearly shown that the intracytoplasmic membrane-proximal tyrosine residue is involved in this polarized budding phenotype of HIV in lymphocytes, as was the case in polarized epithelial MDCK cells. It should be mentioned that the presence of a tyrosine-based signal involved in the polarized budding of SIV in lymphocytes had also been previously suggested. In this case, the polarization of the envelope glycoprotein at the cell surface was affected when the membrane-proximal tyrosine 723 residue was replaced by another amino acid (24), and the resulting viruses appeared to be less pathogenic (21). In the last few years, similar tyrosine-based signals have been found in various basolateral proteins and have been associated with various types of targeted protein delivery (5, 20, 22, 30, 31, 33, 51, 52). A tyrosine-based signal has been proposed to be involved in endocytosis of the HIV glycoprotein at the cell surface (47), while this signal is inhibited in the presence of an excess of viral capsid proteins (11). An increase in the amount of envelope glycoproteins at the cell surface with the mutant proviral constructs used in the present study suggests that the endocytosis signal is still functional under conditions of a normal ratio between capsid and envelope proteins. There is thus at least a partial overlap between the signals responsible for basolateral delivery and for endocytosis of HIV-1 envelope glycoprotein at the cell surface, and both signals can be active in lymphocytes. It is suggested that endocytosis maintains limited levels of the viral glycoprotein at the cell surface whereas the signal is inhibited by interaction with capsid protein in the process of viral budding (30, 51).
It remains to be established if both targeted viral budding and endocytosis of envelope glycoprotein from the cell surface are actually important during viral multiplication and/or viral pathogenesis. Observations showing preferential release at intercellular contact zones did suggest a role for polarization in viral transmission from infected to uninfected cells (38, 39). It is generally accepted that the transmission of virus by close contact between infected and uninfected cells is much more efficient than transmission by cell-free viruses. This could play an important role during sexual transmission from infected cells toward the epithelial surface of mucosa.
In the present study, the presence of the polarization signal was shown to favor propagation of the viral infection under conditions where viral propagation by cell-to-cell contact is likely to be predominant. The effect was observed when small numbers of chronically infected cells producing limited amounts of viruses were used, thus mostly avoiding transmission via released cell-free virus. A short delay in viral propagation for the mutant virus was also occasionally observed at very early times in transfection experiments or when infection with cell-free virus was performed at a very low multiplicity of infection, two situations where cell-to-cell propagation would be transiently predominant (data not shown). We believe that cell-to-cell transmission from small numbers of cells producing low levels of virus is representative of a situation that could occur in vivo, especially during sexual transmission, when infected mononuclear cells transmit the virus at mucosal surfaces. This also suggests that the polarized-budding phenotype of HIV could have important consequences during viral propagation in the infected host by favoring transmission from antigen-presenting cells to interacting T lymphocytes. This importance of tyrosine-based signals in retroviral pathogenesis is further supported by analogy to other viruses. For bovine leukemia virus, a tyrosine-based motif was shown to be essential for infection and maintenance of a high viral load in infected animals (59). Furthermore, two of three tyrosine-based motifs in the intracytoplasmic tail of the transmembrane protein are apparently involved in signal transduction pathways (59). This might have significance, considering that cytoskeletal elements in polarized lymphocytes can be colocalized with signal-transducing protein kinase C (17). Also, during T-cell activation, tyrosine phosphorylation of different substrates is induced at the site of polarization. It is thus quite possible that retroviruses harboring tyrosine-based signals can take advantage of a normal cellular process, by which various specific molecules are relocalized to the pseudopod during lymphocyte activation, in order to facilitate an efficient viral transmission from infected to uninfected cells. The polarization signal in HIV could thus become a new therapeutic target to limit cell-to-cell propagation of the virus.
ACKNOWLEDGMENTS
J.D. and J.-P.L. contributed equally to this work.
We thank Serge Sénéchal and Hugo Diluydy for performing the FACS analysis and confocal microscopic examination, respectively, and for their help in interpreting the results. We thank M. Robert Alain from IAF (Institut Armand Frappier) for performing electron microscopic observations and Xian Jian Yao for providing us with purified human PBMCs. We thank Carole Danis, Nicole Rougeau, Isabelle Courchesne, and Johanne Mercier for technical support.
This work was supported by grants from the National HIV Research and Development Program (NHRDP) and Medical Research Council of Canada (MRC) (to G.L. and É.A.C.) as well as a Fonds pour la formation de chercheur et l’aide à la recherche (FCAR) group grant. É.A.C. is the recipient of an MRC scientist award, and G.L. is the recipient of a scholarship from the Fonds de la recherche en santé du Québec (FRSQ). J.P.L. was the recipient of a FCAR studentship through the Groupe de recherche en transport membranaire, J.D. and R.L. were recipients of NHRDP studentships, and G.C.-A. is the recipient of a studentship from the Ministère de l’éducation du Québec.
REFERENCES
- 1.Bourinbaiar A S, Phillips D M. Transmission of human immunodeficiency virus from monocytes to epithelia. J Acquired Immune Defic Syndr. 1991;4:56–63. [PubMed] [Google Scholar]
- 2.Carpén O, Virtanen I, Lehto V P, Saksela E. Polarization of NK cell cytoskeleton upon conjugation with sensitive target cells. J Immunol. 1983;131:2695. [PubMed] [Google Scholar]
- 3.Cohen É, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compans R W, Srinivas R V. Protein sorting in polarized epithelial cells. Curr Top Microbiol Immunol. 1991;170:141–181. doi: 10.1007/978-3-642-76389-2_5. [DOI] [PubMed] [Google Scholar]
- 5.Dagermont C, Le Bivic A, Rothemberger S, Lacopetta B, Kuhn L C. The internalization signal and the phosphorylation site of transferrin receptor are distinct from the main basolateral sorting information. EMBO J. 1993;12:1713–1721. doi: 10.1002/j.1460-2075.1993.tb05816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Hoop M J, Dotti C G. Membrane traffic in polarized neurons in culture. J Cell Sci Suppl. 1993;17:85–92. doi: 10.1242/jcs.1993.supplement_17.13. [DOI] [PubMed] [Google Scholar]
- 7.Delchambre M, Gheysen D, Thine D, Thiriart C, Jacobs E, Verdin E, Horth M, Burny A, Bex F. The gag precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989;13:2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Pozo M A, Sanchez-Mateos P, Nieto M, Sanchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J Cell Biol. 1995;131:495–508. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotti C G, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- 10.Dotti C G, Kartenbeck J, Simons K. Polarized distribution of the viral glycoproteins of vesicular stomatitis, fowl plague and Semliki Forest viruses in hippocampal neurons in culture: a light and electron microscopy study. Brain Res. 1993;610:141–147. doi: 10.1016/0006-8993(93)91227-j. [DOI] [PubMed] [Google Scholar]
- 11.Egan M C, Carruth L M, Rowell J F, Yu X, Siliciano R F. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol. 1996;70:6547–6556. doi: 10.1128/jvi.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fais S, Capobianchi M R, Abbate I, Castilletti C, Gentile M, Fei P C, Ameglio F, Dianzani F. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with co-polarization of intercellular adhesion molecules and HIV-1 viral matrix protein. AIDS. 1995;9:329–335. [PubMed] [Google Scholar]
- 13.Fantini J, Baghdiguian S, Yahi N, Chermann J C. Selected human immunodeficiency virus replicates preferentially through the basolateral surface of differentiated human colon epithelial cells. Virology. 1991;185:904–907. doi: 10.1016/0042-6822(91)90570-2. [DOI] [PubMed] [Google Scholar]
- 14.Fisher A G, Collalti E, Ratner L, Gallo R C, Wong-Staal F A. Molecular clone of HTLV-III with biological activities. Nature. 1985;316:262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- 15.Gabuzda D H, Lever A, Terwilliger E, Sodroski J. Effects of deletions in the cytoplasmic domain in biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1992;66:3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Assembly and release of HIV-1 precursor pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 17.Gregorio C C, Repasky E A, Fowler V M, Black J D. Dynamic properties of ankyrin in T lymphocytes: colocalization with spectrin and protein kinase C beta. J Cell Biol. 1994;125:345–358. doi: 10.1083/jcb.125.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handler J S. Overview of epithelial polarity. Annu Rev Physiol. 1989;51:729–740. doi: 10.1146/annurev.ph.51.030189.003501. [DOI] [PubMed] [Google Scholar]
- 19.Haston W S. F-actin distribution in polymorphonuclear leukocytes. J Cell Sci. 1987;88:495–501. doi: 10.1242/jcs.88.4.495. [DOI] [PubMed] [Google Scholar]
- 20.Honing S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-1) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoxie J A. Proceedings of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. In vivo attenuation of SIVmac239 by mutation of a conserved Tyr in the TM cytoplasmic tail, abstr. 520. [Google Scholar]
- 22.Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1992;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- 23.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaBranche C C, Sauter M M, Haggarty B S, Vance P J, Romano J, Hart T K, Bugelski P J, Marsh M, Hoxie J A. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J Virol. 1995;69:5217–5227. doi: 10.1128/jvi.69.9.5217-5227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M H, Sano K, Morales F E, Imagawa D T. Sensitive reverse transcriptase assay to detect and quantitate human immunodeficiency virus. J Clin Microbiol. 1987;25:1717–1721. doi: 10.1128/jcm.25.9.1717-1721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letourneur, F., and R. A. Klausner. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell 69:1143–1157. [DOI] [PubMed]
- 27.Lodge R, Göttlinger H, Gabuzda D, Cohen É, Lemay G. The intracytoplasmic domain of gp41 mediates polarized budding of human immunodeficiency virus type 1 in MDCK cells. J Virol. 1994;68:4857–4861. doi: 10.1128/jvi.68.8.4857-4861.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodge R, Lalonde J-P, Lemay G, Cohen É. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 1996;16:695–705. doi: 10.1093/emboj/16.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodge R, Subbramanian R A, Forget J, Lemay G, Cohen É. MuLV-based vectors pseudotyped with truncated HIV glycoprotein mediate specific gene transfer in CD4(+) peripheral blood lymphocytes. Gene Ther. 1998;5:655–664. doi: 10.1038/sj.gt.3300646. [DOI] [PubMed] [Google Scholar]
- 30.Marsh M, Pelchen-Matthews A, Hoxie J A. Roles for endocytosis in lentiviral replication. Trends Cell Biol. 1997;7:1–4. doi: 10.1016/S0962-8924(97)20038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 32.McNeill H, Ozawa M, Kemler R, Nelson W J. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- 33.Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyataka S, Saito T, Gailusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 34.Owens R J, Compans R W. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J Virol. 1989;63:978–982. doi: 10.1128/jvi.63.2.978-982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce-Pratt R, Phillips D M. Studies of adhesion of lymphocytic cells: implications for sexual transmission of human immunodeficiency virus. Biol Reprod. 1993;48:431–445. doi: 10.1095/biolreprod48.3.431. [DOI] [PubMed] [Google Scholar]
- 36.Pearce-Pratt R, Malamud D, Phillips D M. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J Virol. 1994;68:2898–2905. doi: 10.1128/jvi.68.5.2898-2905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perotti M-E, Tan X, Phillips D M. Directional budding of human immunodeficiency virus from monocytes. J Virol. 1996;70:5916–5921. doi: 10.1128/jvi.70.9.5916-5921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips, D. M., and A. S. Bourinbaiar. Mechanism of HIV spread from lymphocytes to epithelia. Virology 186:261–273. [DOI] [PubMed]
- 39.Phillips D M, Zacharopoulos V R, Tan X, Pearce-Pratt R. Mechanisms of sexual transmission of HIV: does HIV infect intact epithelia? Trends Microbiol. 1994;2:454–458. doi: 10.1016/0966-842x(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 40.Ratner L, Fisher A, Jagodzinski L L, Mitsuya H, Liou R S, Gallo R C, Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987;3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 41.Rhee S S, Hunter E. Structural role of the matrix protein of type D retroviruses in gag polyprotein stability and capsid assembly. J Virol. 1990;64:4383–4389. doi: 10.1128/jvi.64.9.4383-4389.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Boulan E, Nelson W J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Boulan E, Powell S K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Boulan E, Sabatini D D. Asymmetric budding of viruses in epithelial cell monolayers: a model system for study of epithelial polarity. Proc Natl Acad Sci USA. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez-Boulan E. Membrane biogenesis: enveloped RNA viruses and epithelial polarity. Mol Cell Biol. 1983;1:119–170. [Google Scholar]
- 46.Rosenberg Z F, Fauci A S. Induction of expression of HIV in latently or chronically infected cells. AIDS Res Hum Retroviruses. 1989;5:1–4. doi: 10.1089/aid.1989.5.1. [DOI] [PubMed] [Google Scholar]
- 47.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 48.Sasaki H, Nakamura M, Ohno T, Matsuda Y, Yuda Y, Nonomura Y. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc Natl Acad Sci USA. 1990;92:2026–2030. doi: 10.1073/pnas.92.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauter M M, Pelchenmatthews A, Bron R, Marsh M, Labranche C C, Vance P J, Romano J, Haggarty B S, Hart T K, Lee W M F, Hoxie J A. An internalization signal ln in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J Cell Biol. 1996;132:795–811. doi: 10.1083/jcb.132.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan S J, Daukas G, Zigmond S H. Asymetric distribution of the chemotactic peptide receptor on polymorphonuclear leukocytes. J Cell Biol. 1984;99:1461–1467. doi: 10.1083/jcb.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas D C, Brewer C B, Roth M G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- 52.Thomas D C, Roth M G. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- 53.Tucker S P, Compans R W. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42:187–247. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasiliev, J. M. Polarization of pseudopodial activities. 1991. J. Cell Sci. 98:1–4. [DOI] [PubMed]
- 55.Weclewicz K, Ekström M, Kristensson K, Garoff H. Specific interactions between retrovirus Env and Gag proteins in rat neurons. J Virol. 1998;72:2832–2845. doi: 10.1128/jvi.72.4.2832-2845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkinson P C, Higgins A. OKT3-activated locomotion of human blood lymphocytes: a phenomenon requiring contact of T cells with Fc receptor-bearing cells. Immunology. 1987;60:445–451. [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkinson P C. Leucocyte locomotion: behavioural mechanisms for accumulation. J Cell Sci Suppl. 1987;8:103–119. doi: 10.1242/jcs.1987.supplement_8.6. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson P C. The locomotor capacity of human lymphocytes and its enhancement by cell growth. Immunology. 1986;57:281–289. [PMC free article] [PubMed] [Google Scholar]
- 59.Willems L, Gatot J S, Mammericks M, Portetelle D, Burny A, Kerkhofs P, Kettman R. The YXXL signalling motifs of the bovine leukemia virus transmembrane protein are required for in vivo infection and maintenance of high viral loads. J Virol. 1995;69:4137–4141. doi: 10.1128/jvi.69.7.4137-4141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao X J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen É. Mutagenic analysis of human immunodeficiency virus type 1 VPR: role of predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zacharopoulos V R, Perotti M E, Phillips D M. Lymphocyte-facilitated infection of epithelia by human T-cell lymphotropic virus type I. J Virol. 1992;66:4601–4605. doi: 10.1128/jvi.66.7.4601-4605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]