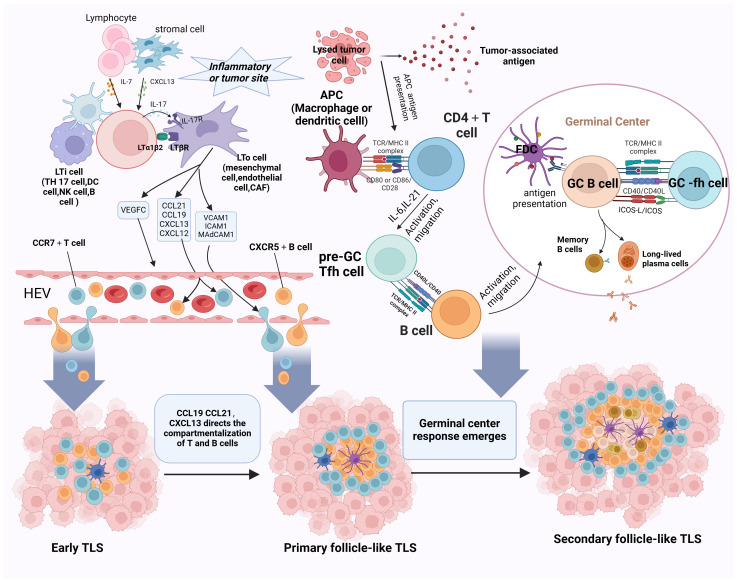
Figure 2.
Schematic representation of the progression of TLS development and maturation. The initiation of TLS formation commences with the release of CXCL13 and IL-7 by stromal cells, which prompts the recruitment of LTi cells into tumor tissues. B cells, Th17 cells, NK cells, and DC cells take on the role of LTi cells. CAFs, mesenchymal cells, and endothelial cells substitute for LTo cells. Paracrine signaling of IL-7 induces LTi cells to express Tα1β2 and bind to LTβR on activated LTo cells. This LTβR signaling stimulates LTo cells to secrete VEGFC, consequently facilitating HEV formation. Additionally, IL-17 secreted by LTi cells binds to IL-17R on LTo cells. The LTα1β2-LTβR and IL-17-IL-17R signaling pathways promote the secretion of adhesion molecules (MADCAM1, ICAM1, and VCAM1) and chemokines (CXCL12, CXCL13, CCL19, and CCL21) by LTo cells, attracting lymphocytes to infiltrate tumor tissues via HEVs, thus initiating the formation of E-TLS. Subsequently, CCL19/CL21 and CXCL13 orchestrate the compartmentalized distribution of CCR7+ T cells and CXCR5+ B cells, respectively, leading to the organized arrangement of TLS and the transition to the primary follicle-like TLS stage. Finally, with the assistance of FDCs and Tfh cells, the germinal center reaction ensues, facilitating SHM, B-cell clonal selection, and the generation of high-affinity antibodies within the TLS. This maturation process culminates in the progression to SFL-TLS. LTi, lymphoid tissue-inducing cell; LTα1β2, lymphotoxin α1β2; LTβR, lymphotoxin β receptor; VEGFC, vascular endothelial growth factor C; CAFs, cancer-associated fibroblasts; SHM, somatic hypermutation. Created with Biorender.com.