Fig. 7.
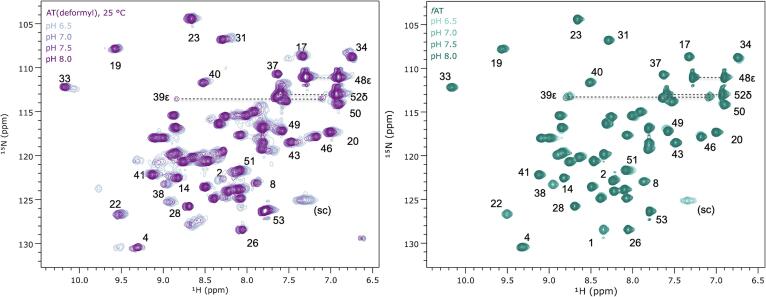
Protonation of the Met1 amine is coupled to oligomerization of AT. Left, 1H-15N correlated spectra of AT recorded at 25 °C and pH 6.5, 7.0, 7.5 and 8.0 reveal strong perturbations of several signals, and line narrowing at high pH, which favors trimeric AT. Right, for fAT the N-terminal amine is protected by the formyl group and very minor shift perturbations are observed only for residues flanking His50; this indicates no pH-dependent structural shifts in the AT trimer. Amide assignments transferred from 55 °C via a temperature series. (sc), side chain amide.