Abstract
Cortical spreading depression (CSD) is a slow wave of cortical depolarization closely associated with migraines with an aura. Previously, it was thought that CSD depolarization was mainly driven by neurons, with characteristic changes in neuronal swelling and increased extracellular potassium (K+) and glutamate. However, the role of astrocytes, a member of the neurovascular unit, in migraine with CSD has recently received increasing attention. In the early stages of CSD, astrocytes provide neurons with energy support and clear K+ and glutamate from synaptic gaps. However, in the late stages of CSD, astrocytes release large amounts of lactic acid to exacerbate hypoxia when the energy demand exceeds the astrocytes’ compensatory capacity. Astrocyte endfoot swelling is a characteristic of CSD, and neurons are not similarly altered. It is primarily due to K+ influx and abnormally active calcium (Ca2+) signaling. Aquaporin 4 (AQP-4) only mediates K+ influx and has little role as an aquaporin. Astrocytes endfoot swelling causes perivascular space closure, slowing the glymphatic system flow and exacerbating neuroinflammation, leading to persistent CSD. Astrocytes are double-edged swords in migraine with CSD and may be potential targets for CSD interventions.
Keywords: Headache, Aura, CSD, Neurovascular unit, Depolarization, Energy metabolism, Inflammatory mediators, Glymphatic system
Key Summary Points
| Cortical spreading depression (CSD) is considered a potential cause of migraines with an aura, but is not exclusive to migraines. Astrocytes are not only supporting cells for neurons, and the role of astrocytes in migraine with CSD has recently received increasing attention. |
| Astrocyte endfoot swelling is a characteristic of CSD, and neurons are not similarly altered. Astrocyte endfoot swelling, resulting in perivascular space closure, affects neuroinflammation clearance by the glymphatic system. |
| Astrocytic endfoot swelling during CSD is primarily due to K+ influx and abnormally active Ca2+ signaling. AQP-4 only mediates the process of K+ influx and plays a little role as an aquaporin. |
| Astrocytes are a double-edged sword, with protective effects on neurons during the early stages of CSD. Astrocytes provide neurons with energy support and clear K+ and glutamate from synaptic gaps. With CSD progression, astrocyte dysfunction drives the CSD spread. |
Introduction
Cortical spreading depression (CSD) is associated with many diseases, including migraine, trauma, cerebrovascular disease, and mitochondrial disease [1]. The relationship between migraines and CSD has been extensively studied. There are many migraine types, but the most widely studied are those with and without an aura [2]. CSD is considered a potential cause of migraines with an aura [3], and was first described by Leao [4]. This depolarizing wave usually spreads slowly through the cortex at 2–5 ms/min [5]. The mechanism of CSD generation is unclear, and it was initially thought to be associated with calcium (Ca2+). However, it has been shown that astrocytic Ca2+ wave inhibition did not hinder the spread of CSD, suggesting that Ca2+ waves are not involved in CSD depolarization [6]. It is now widely recognized that CSD may be associated with the rapid influx of cations and disturbed sodium–potassium adenosine triphosphatase (Na+/K+-ATPase) activity. The difference between the intra- and extracellular ion concentrations is maintained by the Na+/K+-ATPase [7]. Ionic homeostasis restoration after CSD is achieved by increasing its activity [8]. Sufficient energy is required to maintain the Na+/K+-ATPase, and therefore insufficient brain energy may impair its activity, thereby lowering the CSD threshold [9]. Potassium chloride (KCl) is widely used in CSD animal experiments to increase extracellular K+ concentration to initiate CSD [6]. Mouse models of familial hemiplegic migraine type 1 (FHM1, excessive inter-synaptic glutamate release), FHM2 (reduced inter-synaptic K+ and glutamate scavenging), and FHM3 (Na+ channel inactivation) have significantly lower CSD thresholds [10–12]. The FHM2 mouse model has been extensively studied owing to its increased susceptibility to CSD. FHM2 is caused by mutations in α2 Na+/K+-ATPase (α2NKA), expressed mainly in astrocytes [13], while α2NKA is vital in K+ clearance during neuronal activity [14]. Astrocytic excitatory amino acid transporter 2 (EAAT2) was decreased by 50% in FHM2 knockout mice compared with that in wild-type mice [15]. Excitatory amino acid transporter 1 (EAAT1) and EAAT2 co-localize with the α2NKA, and the clearance of glutamate by these two transporters is dependent on the Na+ gradient, which is maintained by the α2NKA [13]. Upregulating astrocytic EAAT2 alleviates migraine in a rat model of chronic migraine [16].
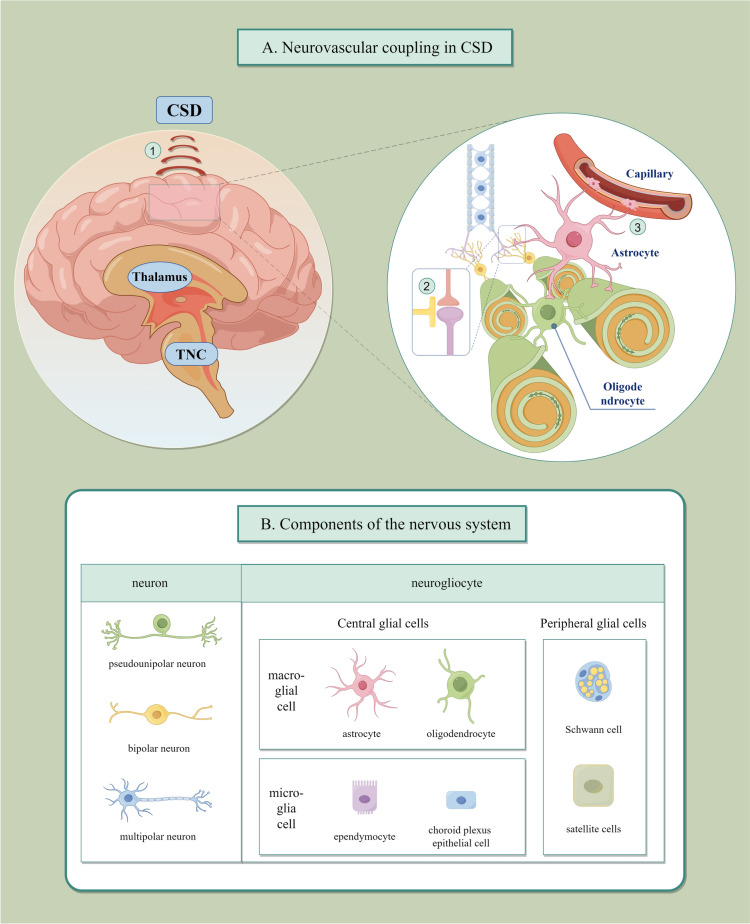
Migraine with an aura is associated with cortical hemodynamic changes, as shown by a persistent decrease in cerebral blood flow (CBF) that coincides with headaches [17]. CSD is characterized by neuronal swelling and elevated extracellular potassium and glutamate levels [18]. CSD is a metabolic stress event with an extremely high energy demand that depletes glucose and decreases ATP concentration by approximately 50% [7]. This process may activate the neurovascular unit (NVU) (Fig. 1A). The NVU comprises many cell types, including astrocyte endfeet, parenchymal neuronal projections, perivascular nerves, pericytes, smooth muscle cells, and endothelium [19]. The NVU can couple neural activity with CBF, and when the NVU is tightly coupled, the brain lacks energy and must promptly deliver oxygen and glucose to high metabolic areas [20]. NVU activation is accompanied by secondary CBF changes, shown by transient hypoperfusion in the first stage, significant primary hyperemia (vasodilation) in the second stage, mild hyperemia in the third stage, and persistent hypoxemia in the fourth stage [21, 22]. The role of astrocytes, a member of the NVU, in migraine with CSD has recently received increasing attention. This review article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Fig. 1.
Cortical spreading depression (CSD) and neurovascular unit (NVU). (A) CSD occurs primarily in the cerebral cortex (1) and involves complex electrophysiological activity, as shown by the inter-synaptic transport of lactate, ions, and amino acids between neurons and astrocytes (2). CSD activates the NVU (3). Astrocyte endfeet is a component of the NVU, which has strong physiological regulatory effects, including regulating cerebral blood flow, maintaining the blood–brain barrier function, and participating in the glymphatic system. (B) Neurogliocytes are divided into central and peripheral glial cells, subdivided into macroglia and microglia. Astrocytes are a type of macroglia with a star-shaped appearance
Astrocytes: Not Only Supporting Cells for Neurons
The adult central nervous system cells can be divided into neurons and neurogliocytes [23]. The latter mainly comprises microglias, oligodendrocytes, and astrocytes (Fig. 1B). Microglias are multifunctional macrophages that settle in the brain, and astrocytes are considered neuronal support cells [24, 25]. Astrocytes originate from neural stem cells in the subventricular zone during development and migrate along radial glial cell projections into the central nervous system (CNS) [26]. Astrocytes are the most abundant cell type in the mammalian brain, accounting for approximately 20–40% of the brain cell [27]. They are highly heterogeneous. Heterogeneity can also be detected within the same CNS region [23]. Astrocytes primarily comprise glial fibrillary acidic protein (GFAP). GFAP expression is upregulated in astrocytes after neurological injury and can be used as a marker of astrocyte activation [23, 28]. Another standard marker for astrocytes is the S100β protein. It is a class of Ca2+-binding proteins widely distributed in neural tissues that act as an axonal growth factor [24]. Serum S100β levels are increased after acute cerebral ischemia in rats; therefore, it can also be used as a biochemical marker after brain injury [29].
Astrocytes have more extensive gap junctions, also known as nexuses, than any other type of brain cells, consisting of many connexons arranged in a regular plate-like pattern [24]. Each connexon comprises six subunits called connexins (Cx). The gap junction is a direct channel for intercellular signaling. Astrocytes predominantly express Cx43 and, to a lesser extent, Cx30 [30]. Astrocytes take up excess potassium ions released into the extracellular space during neuronal excitation and diffuse rapidly through intercellular gap junctions, preventing the significant rise of K+ concentration around excitable neurons. Traditionally, astrocytes have been widely distributed, and they encircle neurons to support and isolate them [25]. However, an astrocyte has recently been shown to continuously exchange information with multiple neurons and is vital in the development, normal physiology, and pathology of the CNS [31]. The abundant gap junctions and multiple ion channels in astrocytes can regulate the ion concentration and pH inside and outside neurons, mainly by controlling the extracellular K+ concentration to maintain the stability of the internal environment [24]. Astrocytes have Na+/K+ ATPase and Na+/K+/2CI- transporters to promptly pump K+ into the cell and Na+ out of the cell. They also have aquaporin-4 (AQP-4) to co-regulate ionic balance and pH homeostasis [13, 32].
The Role of Astrocytes in Cortical Spreading Depression
Previous studies have shown that CSD depolarization is mainly driven by neurons [19]. Astrocytes follow only passively, and changes in astrocytic Ca2+ waves lag behind neurons; however, Ca2+ waves are not responsible for generating and spreading CSD depolarization under most conditions [33]. Therefore, this does not indicate passive changes in astrocytes during CSD. CSD is also more difficult to evoke in brains with a higher ratio of glial cells to neurons, and increasing the density of astrocytes slows the spread of CSD waves [18, 34]. This suggests that astrocytes’ role in patients with CSD is non-negligible.
Astrocytes Provide Energy Support for Neurons
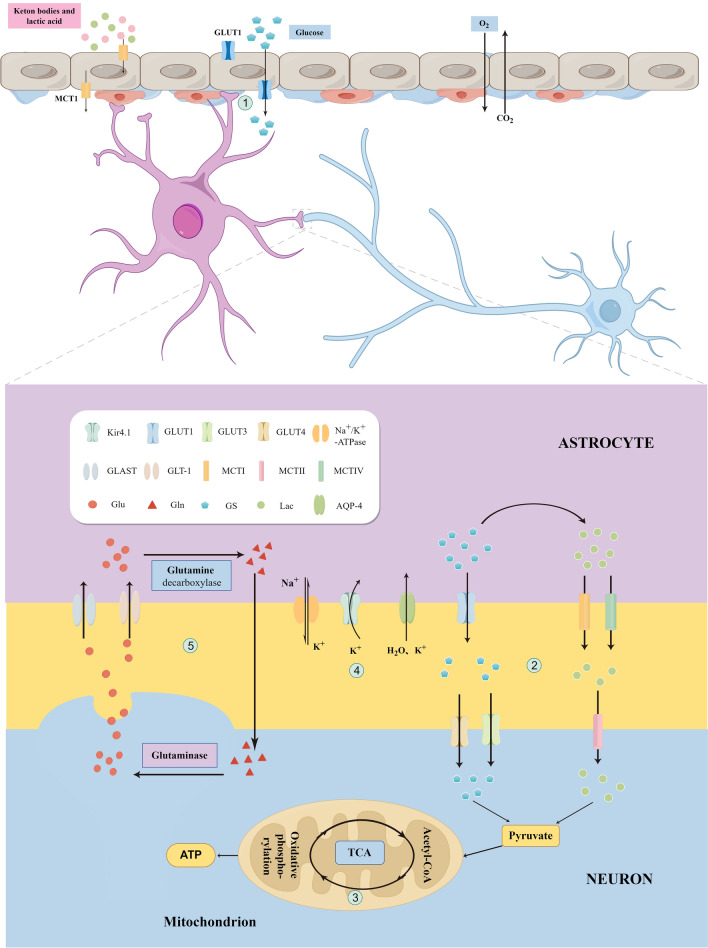
As CNS glycolytic cells, astrocytes provide energy metabolism substrates for neurons and oligodendrocytes, control extracellular water and electrolyte homeostasis, and regulate metabolic homeostasis by synthesizing glycogen and providing energy to neurons [24]. Glucose is the brain’s primary energy source but rarely directly enters the neurons [35]. It first passes through the blood–brain barrier into astrocytes, where it is processed and eventually transported to neurons as lactic acid. Astrocytes support oxidative metabolism in neurons [36]. The astrocyte–neuron lactate shuttle enables the energetic coupling between the two [37, 38]. Glucose crosses the endothelium from the arterial blood into the brain cells through isoforms of glucose transporters (GLTs) [38, 39]. GLT1 is predominantly present in endothelial cells and astrocytes, whereas GLT3 and GLT4 are mainly expressed in neurons [40]. Glucose enters astrocytes and is enzymatically cleaved to lactate, which is then transported to neurons for energy support through sequential processing by astrocytic I and IV monocarboxylate transporters (MCTI/IV) and neuronal MCTII (Fig. 2). Neurons contain enzymes essential for glycogen metabolism, particularly glycogen synthase, which is permanently degraded by the proteasome system [39]. Consequently, the rate of lactate uptake by neurons is seven-fold higher than that of glucose [41, 42].
Fig. 2.
Synaptic physiologic activity of neurons and astrocytes in cortical spreading depression (CSD). Glucose transporter 1 (GLT1) is primarily expressed in the endothelial cells and astrocytes. Glucose enters astrocytes via GLT1 through the blood–brain barrier (Step 1) and undergoes oxidative metabolism to produce lactate (Step 2). Lactate enters the neurons through a monocarboxylate transporter (MCT) to produce pyruvate, which undergoes oxidative decarboxylation to form acetyl coenzyme A, entering the tricarboxylic acid cycle (Step 3). Synaptic gap excess K+ influxes into astrocytes during CSD (Step 4), leading to swelling of astrocyte endfeet. The massive release of glutamate (Glu) from neurons during CSD enters astrocytes through Glu transporter 1 (GLT-1) and Glu aspartate transporter (GLAST) to generate glutamine (Gln). Gln leaves astrocytes by exocytosis, enters the neuron by endocytosis, and is converted to Glu for its release into the synaptic gap in a process known as the Glu–Gln cycle (Step 5)
Hyperglycemia increases the CSD threshold and decreases the frequency of CSD events. In contrast, hypoglycemia prolongs CSD duration and decreases the KCl-induced CSD threshold by over 50% [23]. In the absence of energy in patients with CSD, astrocytes provide the main energy source by upregulating GLTs and catabolizing glycogen, leaving 80% of blood-derived glucose in neurons [8, 18]. In addition, astrocytes promote glycolysis during CSD recovery, as evidenced by decreased astrocytic pH during CSD, which coincides with accelerated glycolysis [33]. However, the accumulation of large amounts of lactic acid in the late stages of CSD leads to vasoconstriction and increased tissue hypoxia, which inhibits mitochondrial respiration in astrocytes, leading to mitochondrial depolarization, free radical generation, lipid peroxidation, and intracellular calcium ion release [43].
Astrocytes Clear Synaptic Gap K+ and Glutamate
The most critical role of astrocytes is to clear the synaptic gap between K+ and glutamate (Glu), both of which are released outside the cell during neuronal activity [44]. The inwardly rectifying K+ channel (Kir4.1) is highly expressed in astrocytes and is responsible for scavenging the large amounts of K+ released during neuronal activity [26]. Glu is the brain’s most vital excitatory amino acid; it stimulates glycolysis and promotes lactate production, and its intake is closely associated with energy metabolism [45]. Glu uptake requires glycogen even when the glucose supply is adequate [46]. The brain expresses five amino acid transport proteins [47]. Astrocytes predominantly express EAAT1, the GLAST, and EAAT2, the GLT-1. Glu released into the synaptic gap by neurons is taken up by astrocytes through EAAT1 and EAAT2 [38]. EAAT2 is a scavenger responsible for 90% of Glu uptake [16]. Glu is taken up by astrocytes and converted into glutamine (Gln) by glutamine decarboxylase, which is then converted to Gln by glutaminase in the neurons [48]. This process is known as the Glu–Gln cycle [32, 38]. Glu uptake and inactivation by astrocytes limit excitotoxic effects, providing neuronal protection (Fig. 2). In the early stages of CSD, astrocytes limit the spread of CSD by increasing Na+/K+-ATPase activity, thereby transporting excess extracellular K+ into the cells [8]. In addition, the astrocyte Cx43 reduces extracellular K+ and Glu levels [49]. This is consistent with the results observed in mouse brain sections by Theis et al. [50]. Impaired K+ redistribution in Cx43-deficient mice manifests as a sudden increase in localized K+ and accelerated CSD spread; however, astrocyte gap junctions play only a partial role in K+ buffering/redistribution. Breithausen et al. [51] also suggested that astrocyte gap junctions have a limited role in extracellular K+ buffering. Na+/K+ATPase impairment increases extracellular potassium and intracellular sodium, leads to massive Glu release, and impairs astrocyte clearance [7], resulting in persistent CSD. Electrophysiological and hemodynamic analyses were performed on knockout mice to assess CSD susceptibility and to investigate the correlation between GLT and CSD [52]. The results showed significant CSD frequency and rate changes only in GLT-1 knockout mice. This indicates that astrocyte dysfunction affects glutamate clearance and that large extracellular accumulation of glutamate is essential in producing persistent CSD [53].
Proliferation of Astrocytes Releases Inflammatory Mediators
CSD may activate meningeal nociceptors, which in turn activate nuclear factor-κB (NF-κB) in astrocytes by opening neuronal Pannexin-1 (Panx1) channels and caspase-1 [54]. Cyclooxygenase-2 (COX-2) is subsequently released into the subarachnoid space and triggers inducible nitric oxide synthase, producing an inflammatory cascade [41]. CSD spreads through the activation of macrophages and mastocytes and the subsequent release of different inflammatory mediators to induce localized neuroinflammation [55]. The selective COX-2 inhibitor, celecoxib, reduces the CSD-induced activation of dural macrophages in rodents [56].
CSD-induced astrocyte proliferation, reactive astrocyte proliferation, is an early response to injury. It induces proliferation, hypertrophy, downregulation of GLT, and the release of the glial medium [16, 57]. Indoleamine-2,3-dioxygenase, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β immunoreactivity were significantly increased after repeated CSD [58]. This suggests that CSD leads to the reactive proliferation of astrocytes, which promotes brain inflammation through mediators such as cytokines. Inflammatory factor TNF-α alters the synthesis and stability of the GLT-1 transporter on astrocytes by upregulating mRNA and protein expression of GLT-1α and GLT-1β shear variants [47]. Impaired inter-synaptic Glu uptake, the accumulation of inflammatory factors, and extracellular Glu contributed to CSD spread (Fig. 3).
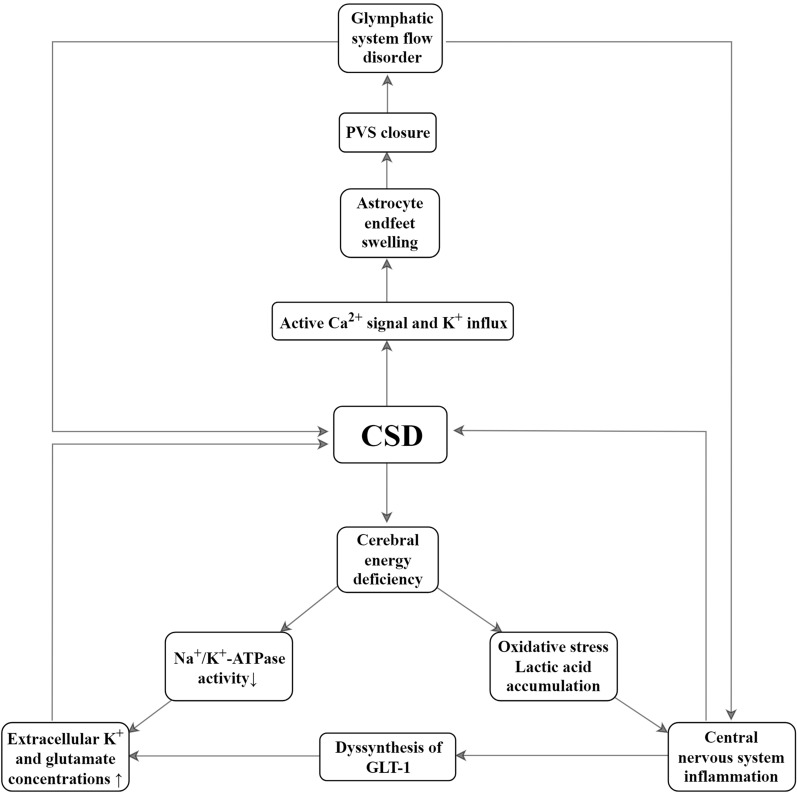
Fig. 3.
Role of astrocytes in cortical spreading depression (CSD). Overactive Ca2+ signaling and K+ influx are responsible for the swelling of astrocytic endfeet. Endfoot swelling causes perivascular space (PVS) closure, slows glymphatic system flow, and exacerbates neuroinflammation. Tumor necrosis factor α (TNF-α) impairs glutamate transporter 1 (GLT-1) synthesis and exacerbates glutamate accumulation in the synaptic gap, leading to persistent CSD.
Astrocyte Endfeet Swelling
The astrocytic body gives off many long branches that fill the space between nerve cells and separate them. The ends of the astrocyte bodies were inflated to form the endfeet [38]. Notably, some endfeet, called vascular or perivascular endfeet, are attached to the walls of neighboring blood vessels to control blood flow [24, 59]. The other part of the endfoot is in contact with neurons, sensing neuronal activity and transporting nutrients [24, 38]. Cellular swelling and altered polyphasic blood flow are characteristic changes in CSD. Increased size and number of astrocytes have been observed in rat brain slices during CSD, whereas neurons do not undergo similar changes [58]. Perivascular spaces (PVS) are outlets for metabolites produced by the glymphatic system to remove the interstitial fluid (ISF), including excitatory and inflammatory substances generated after CSD. They regulate the width of the PVS, thereby altering the ISF flow rate and composition [60]. As mentioned earlier, insufficient astrocyte energy triggers oxidative stress, which at a certain level releases large amounts of inflammatory factors [61]. Glymphatic system dysfunction exacerbates neurologic inflammation [62]. Glymphatic system plays a significant role in the scavenging of reactive oxygen species (ROS). Excess ROS promote the pro-inflammatory cytokines production by microglia. Glymphatic system dysfunction results in a massive accumulation of pro-inflammatory cytokines, leading to astrocyte hypertrophy and activation, which exacerbates glymphatic system dysfunction and perpetuates a vicious cycle [63]. Excessive amounts of pro-inflammatory factors activate neurons and injure receptors resulting in migraine [64]. Glymphatic system removes brain metabolites such as β-amyloid (Aβ). Glymphatic system dysfunction leads to an overaccumulation of Aβ leading to cognitive impairment [65]. However, the relationship between migraine and cognitive impairment still needs further study.
In addition, the glymphatic system flow depends on AQP-4 in astrocyte endfeet [66]. PVS closure during CSD is due to CSD-induced changes in vessel diameter [67]. It is more likely to be secondary to astrocyte endfoot swelling, as astrocyte endfeet form the outer wall of the PVS [68]. It has been suggested that endfoot swelling may be associated with active Ca2+ signaling, activating proteases, and disrupting molecules closely associated with endfeet function [69]. Ca2+ signaling in astrocytes during CSD depends on AQP-4 [70], and K+ clearance is reduced in AQP-4-deficient mice during CSD [71]. This suggests that AQP-4 maintains K+ homeostasis and acts as an aquaporin [72]. The absence of AQP-4 as an aquaporin had a negligible effect on endfoot swelling during CSD, suggesting that water entry into astrocytes occurs through membrane proteins other than AQP-4 [60]. It has been demonstrated that endfoot swelling during astrocyte CSD is due to K+ influx, which can occur through Kir4.1 and AQP-4 [60, 70]. Astrocytic endfoot swelling during CSD is primarily due to K+ influx and abnormally active Ca2+ signaling. AQP-4 only mediates the process of K+ influx and plays a little role as an aquaporin.
Conclusion
CSD is a strong metabolic stress event (Fig. 3). Astrocyte energy deficiency leads to Na+/K+ ATPase impairment and extracellular K+ accumulation, exacerbating neuronal depolarization and Glu release. Insufficient astrocyte energy triggers oxidative stress, which at a certain level releases large amounts of inflammatory factors. Conversely, CSD leads to astrocyte endfoot swelling, resulting in perivascular space closure, which affects neuroinflammation clearance by the glymphatic system. In conclusion, astrocytes are a double-edged sword, with protective effects on neurons during the early stages of CSD. However, with CSD progression, astrocyte dysfunction drives the CSD spread. Astrocytes may be potential targets for CSD interventions.
Acknowledgments
Medical Writing/Editorial Assistance
The images in this article were drawn by Figdraw (http://www.figdraw.com). We would like to thank Editage (http://www.editage.com) for English language editing.
Author Contributions
All authors contributed to the review. All authors read and approved the final manuscript.
Funding
The Rapid Service Fee was funded by the author (Sui-yi Xu) from Shanxi Basic Research Program (202303021221222).
Declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
This review article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Shah NH, Adams D. Episodic aphasia associated with cortical spreading depression after subdural hemorrhage evacuation. Neurohospitalist. 2016;6(1):1–4. doi: 10.1177/1941874415583118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1): 1–211. [DOI] [PubMed]
- 3.Zhao J, Blaeser AS, Levy D. Astrocytes mediate migraine-related intracranial meningeal mechanical hypersensitivity. Pain. 2021;162(9):2386–2396. doi: 10.1097/j.pain.0000000000002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leão AA. Spreading depression. Funct Neurol. 1986;1(4):363–366. [PubMed] [Google Scholar]
- 5.Close LN, et al. Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia. 2019;39(3):428–434. doi: 10.1177/0333102418774299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles A, Brennan K. Cortical spreading depression-new insights and persistent questions. Cephalalgia. 2009;29(10):1115–1124. doi: 10.1111/j.1468-2982.2009.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borkum JM. Brain energy deficit as a source of oxidative stress in migraine: a molecular basis for migraine susceptibility. Neurochem Res. 2021;46(8):1913–1932. doi: 10.1007/s11064-021-03335-9. [DOI] [PubMed] [Google Scholar]
- 8.Feuerstein D, et al. Regulation of cerebral metabolism during cortical spreading depression. J Cereb Blood Flow Metab. 2016;36(11):1965–1977. doi: 10.1177/0271678X15612779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, et al. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res. 2013;38(3):472–485. doi: 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto H, et al. Astrocytes in Atp1a2-deficient heterozygous mice exhibit hyperactivity after induction of cortical spreading depression. FEBS Open Bio. 2020;10(6):1031–1043. doi: 10.1002/2211-5463.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unekawa M, et al. Enhanced susceptibility to cortical spreading depression in two types of Na(+), K(+)-ATPase α2 subunit-deficient mice as a model of familial hemiplegic migraine 2. Cephalalgia. 2018;38(9):1515–1524. doi: 10.1177/0333102417738249. [DOI] [PubMed] [Google Scholar]
- 12.Auffenberg E, et al. Hyperexcitable interneurons trigger cortical spreading depression in an Scn1a migraine model. J Clin Investig. 2021;131(21):e142202. doi: 10.1172/JCI142202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kros L, Lykke-Hartmann K, Khodakhah K. Increased susceptibility to cortical spreading depression and epileptiform activity in a mouse model for FHM2. Sci Rep. 2018;8(1):16959. doi: 10.1038/s41598-018-35285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capuani C, et al. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med. 2016;8(8):967–986. doi: 10.15252/emmm.201505944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker PD, et al. Non-canonical glutamate signaling in a genetic model of migraine with aura. Neuron. 2021;109(4):611–628.e8. doi: 10.1016/j.neuron.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, et al. Up-regulation of astrocyte excitatory amino acid transporter 2 alleviates central sensitization in a rat model of chronic migraine. J Neurochem. 2020;155(4):370–389. doi: 10.1111/jnc.14944. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Levy D. Dissociation between CSD-evoked metabolic perturbations and meningeal afferent activation and sensitization: implications for mechanisms of migraine headache onset. J Neurosci. 2018;38(22):5053–5066. doi: 10.1523/JNEUROSCI.0115-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrente D, et al. Cortical spreading depression in traumatic brain injuries: is there a role for astrocytes? Neurosci Lett. 2014;565:2–6. doi: 10.1016/j.neulet.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 19.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev. 2015;95(3):953–993. doi: 10.1152/physrev.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tóth R, et al. Astrocyte Ca(2+) waves and subsequent non-synchronized Ca(2+) oscillations coincide with arteriole diameter changes in response to spreading depolarization. Int J Mol Sci. 2021;22(7):3442. doi: 10.3390/ijms22073442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, et al. Regional temperature and quantitative cerebral blood flow responses to cortical spreading depolarization in the rat. J Cereb Blood Flow Metab. 2017;37(5):1634–1640. doi: 10.1177/0271678X16667131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannoni F, Quintana FJ. The role of astrocytes in CNS inflammation. Trends Immunol. 2020;41(9):805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98(1):239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, et al. Energy metabolic disorder of astrocytes may be an inducer of migraine attack. Brain Sci. 2022;12(7):844. doi: 10.3390/brainsci12070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linnerbauer M, Rothhammer V. Protective functions of reactive astrocytes following central nervous system insult. Front Immunol. 2020;11:573256. doi: 10.3389/fimmu.2020.573256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou B, Zuo YX, Jiang RT. Astrocyte morphology: diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther. 2019;25(6):665–673. doi: 10.1111/cns.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasile F, Dossi E, Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. 2017;222(5):2017–2029. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, et al. Protection against acute cerebral ischemia/reperfusion injury by Leonuri Herba Total Alkali via modulation of BDNF-TrKB-PI3K/Akt signaling pathway in rats. Biomed Pharmacother. 2021;133:111021. doi: 10.1016/j.biopha.2020.111021. [DOI] [PubMed] [Google Scholar]
- 30.Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache. 2016;56(1):12–35. doi: 10.1111/head.12725. [DOI] [PubMed] [Google Scholar]
- 31.Carter SF, et al. Astrocyte biomarkers in Alzheimer’s disease. Trends Mol Med. 2019;25(2):77–95. doi: 10.1016/j.molmed.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Rovegno M, Sáez JC. Role of astrocyte connexin hemichannels in cortical spreading depression. Biochim Biophys Acta Biomembr. 2018;1860(1):216–223. doi: 10.1016/j.bbamem.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Seidel JL, et al. Multifaceted roles for astrocytes in spreading depolarization: a target for limiting spreading depolarization in acute brain injury? Glia. 2016;64(1):5–20. doi: 10.1002/glia.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita S, et al. Cytoarchitecture-dependent decrease in propagation velocity of cortical spreading depression in the rat insular cortex revealed by optical imaging. Cereb Cortex. 2016;26(4):1580–1589. doi: 10.1093/cercor/bhu336. [DOI] [PubMed] [Google Scholar]
- 35.Boison D, Steinhäuser C. Epilepsy and astrocyte energy metabolism. Glia. 2018;66(6):1235–1243. doi: 10.1002/glia.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grech O, et al. Alterations in metabolic flux in migraine and the translational relevance. J Headache Pain. 2022;23(1):127. doi: 10.1186/s10194-022-01494-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan SA, et al. Therapeutic implications of altered energy metabolism in migraine: a state-of-the-art review. Cureus. 2020;12(6):e8571. doi: 10.7759/cureus.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dienel GA. Brain glucose metabolism: integration of energetics with function. Physiol Rev. 2019;99(1):949–1045. doi: 10.1152/physrev.00062.2017. [DOI] [PubMed] [Google Scholar]
- 39.Petit JM, et al. Brain glycogen metabolism: a possible link between sleep disturbances, headache and depression. Sleep Med Rev. 2021;59:101449. doi: 10.1016/j.smrv.2021.101449. [DOI] [PubMed] [Google Scholar]
- 40.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19(4):235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 41.Del Moro L, et al. Migraine, brain glucose metabolism and the "neuroenergetic" hypothesis: a scoping review. J Pain. 2022;23(8):1294–1317. doi: 10.1016/j.jpain.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, et al. Sodium butyrate ameliorates the cognitive impairment of Alzheimer's disease by regulating the metabolism of astrocytes. Psychopharmacology. 2022;239(1):215–227. doi: 10.1007/s00213-021-06025-0. [DOI] [PubMed] [Google Scholar]
- 43.Gross EC, et al. The metabolic face of migraine—from pathophysiology to treatment. Nat Rev Neurol. 2019;15(11):627–643. doi: 10.1038/s41582-019-0255-4. [DOI] [PubMed] [Google Scholar]
- 44.Rimmele TS, et al. Extracellular potassium and glutamate interact to modulate mitochondria in astrocytes. ACS Chem Neurosci. 2018;9(8):2009–2015. doi: 10.1021/acschemneuro.8b00124. [DOI] [PubMed] [Google Scholar]
- 45.Fila M, et al. Nutrients to Improve mitochondrial function to reduce brain energy deficit and oxidative stress in migraine. Nutrients. 2021;13(12):4433. doi: 10.3390/nu13124433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilic K, et al. Inadequate brain glycogen or sleep increases spreading depression susceptibility. Ann Neurol. 2018;83(1):61–73. doi: 10.1002/ana.25122. [DOI] [PubMed] [Google Scholar]
- 47.Dumont AO, et al. Differential regulation of glutamate transporter subtypes by pro-inflammatory cytokine TNF-α in cortical astrocytes from a rat model of amyotrophic lateral sclerosis. PLoS One. 2014;9(5):e97649. doi: 10.1371/journal.pone.0097649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Z, et al. A study on the correlation of the asymmetric regulation between the periaqueductal gray and the bilateral trigeminal nucleus caudalis in migraine male rats. J Headache Pain. 2023;24(1):27. doi: 10.1186/s10194-023-01559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Díaz EF, et al. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes. Glia. 2019;67(8):1598–1619. doi: 10.1002/glia.23631. [DOI] [PubMed] [Google Scholar]
- 50.EbrahimAmini A, et al. Neocortical in vivo focal and spreading potassium responses and the influence of astrocytic gap junctional coupling. Neurobiol Dis. 2021;147:105160. doi: 10.1016/j.nbd.2020.105160. [DOI] [PubMed] [Google Scholar]
- 51.Breithausen B, et al. Limited contribution of astroglial gap junction coupling to buffering of extracellular K(+) in CA1 stratum radiatum. Glia. 2020;68(5):918–931. doi: 10.1002/glia.23751. [DOI] [PubMed] [Google Scholar]
- 52.Aizawa H, et al. Glial glutamate transporter GLT-1 determines susceptibility to spreading depression in the mouse cerebral cortex. Glia. 2020;68(12):2631–2642. doi: 10.1002/glia.23874. [DOI] [PubMed] [Google Scholar]
- 53.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267(1–2):3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 54.Karatas H, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 55.Ghaemi A, et al. Immunomodulatory effect of toll-like receptor-3 ligand Poly I:C on cortical spreading depression. Mol Neurobiol. 2016;53(1):143–154. doi: 10.1007/s12035-014-8995-z. [DOI] [PubMed] [Google Scholar]
- 56.Schain AJ, et al. Celecoxib reduces cortical spreading depression-induced macrophage activation and dilatation of dural but not pial arteries in rodents: implications for mechanism of action in terminating migraine attacks. Pain. 2020;161(5):1019–1026. doi: 10.1097/j.pain.0000000000001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni XC, et al. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022;54:102363. doi: 10.1016/j.redox.2022.102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghaemi A, et al. Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia. 2018;38(4):626–638. doi: 10.1177/0333102417702132. [DOI] [PubMed] [Google Scholar]
- 59.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol. 2015;7(9):a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosic B, et al. Aquaporin-4-independent volume dynamics of astroglial endfeet during cortical spreading depression. Glia. 2019;67(6):1113–1121. doi: 10.1002/glia.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latif K, et al. Bergapten attenuates nitroglycerin-induced migraine headaches through inhibition of oxidative stress and inflammatory mediators. ACS Chem Neurosci. 2021;12(18):3303–3313. doi: 10.1021/acschemneuro.1c00146. [DOI] [PubMed] [Google Scholar]
- 62.Mogensen FL, Delle C, Nedergaard M. The glymphatic system (en)during inflammation. Int J Mol Sci. 2021;22(14):7491. doi: 10.3390/ijms22147491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu S, et al. Glymphatic dysfunction induced oxidative stress and neuro-inflammation in major depression disorders. Antioxidants (Basel) 2022;11(11):2296. doi: 10.3390/antiox11112296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vittorini MG, et al. The glymphatic system in migraine and other headaches. J Headache Pain. 2024;25(1):34. doi: 10.1186/s10194-024-01741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gouveia-Freitas K, Bastos-Leite AJ. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021;63(10):1581–1597. doi: 10.1007/s00234-021-02718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang W, et al. Glymphatic dysfunction in migraine mice model. Neuroscience. 2023;528:64–74. doi: 10.1016/j.neuroscience.2023.07.027. [DOI] [PubMed] [Google Scholar]
- 67.Schain AJ, et al. Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci. 2017;37(11):2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jessen NA, et al. The glymphatic system: a beginner's guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szokol K, et al. Augmentation of Ca(2+) signaling in astrocytic endfeet in the latent phase of temporal lobe epilepsy. Front Cell Neurosci. 2015;9:49. doi: 10.3389/fncel.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Enger R, et al. Deletion of aquaporin-4 curtails extracellular glutamate elevation in cortical spreading depression in awake mice. Cereb Cortex. 2017;27(1):24–33. doi: 10.1093/cercor/bhw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao X, et al. Aquaporin-4 regulates the velocity and frequency of cortical spreading depression in mice. Glia. 2015;63(10):1860–1869. doi: 10.1002/glia.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li YK, et al. Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter-1 expression. Neuropsychopharmacology. 2012;37(8):1867–1878. doi: 10.1038/npp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]