Abstract

Suicide gene therapy is a promising strategy for the potential treatment of hepatocellular carcinoma (HCC). However, the lack of high transduction efficiency and targeted vectors in delivering the suicide genes to only the HCC cells is a major impediment. In the present study, we utilized an adeno-associated virus serotype 6 (AAV6) and its exosomal counterpart (exo-AAV) comprising of an inducible Caspase 9 (iCasp9) gene under the control of different promoter systems for targeting HCC cells. We employed a ubiquitous cytomegalovirus immediate early enhancer/chicken β actin promoter (CAG), a liver-specific promoter (LP1), and a baculoviral IAP repeat-containing protein 5 (BIRC5) promoter for liver and cancer cell-specific expression of iCasp9, respectively. We further evaluated these vectors in Huh7 cells for their ability to kill the target cells. BIRC5 and LP1 promoter-driven iCasp9 vectors demonstrated superior cytotoxicity when compared to CAG promoter-driven iCasp9 vectors. Further validation in a murine model of HCC demonstrated that the LP1-iCasp9 or Birc5-iCasp9-based AAV6 vectors contributed to tumor regression (∼2 fold) as effectively as the AAV6-CAG-iCasp9 vectors (∼1.9 fold). Similarly, exo-AAV6 vectors showed ∼2.1 to 2.8 fold superior in vivo tumor regression when compared to mock-treated animals. Our study has developed two novel promoters (LP1 or BIRC5) whose efficacy is comparable to a strong ubiquitous promoter in both AAV and exo-AAV systems. This expands the toolkit of AAV vectors for safe and effective treatment of HCC.
Introduction
Hepatocellular carcinoma (HCC) ranks as the third most frequent cause of mortality in cancer patients.1 Surgery, chemotherapy, and radiotherapy are still the standard of care for HCC treatment.2−4 However, aggressive characteristics of the disease, such as resistance to chemo- or radiotherapy,5,6 and a high incidence of metastasis in HCC7,8 lead to a higher recurrence rate even after tumor removal.9,10 Thus, ideal therapeutic strategies are still being explored. Gene therapy has rapidly evolved as a viable strategy for the potential treatment of cancer by employing various vectors.11,12 Adeno-associated virus (AAV) has emerged as the vector of choice for gene transfer among these vectors due to its beneficial characteristics, including wide tissue tropism, the capacity to infect both dividing and nondividing cells, low immune response, and long-term transgene expression in target cells.13,14 However, some challenges, such as the need for a high vector dose for effective therapy and high production costs of the vectors, remain. Therefore, strategies that can increase the yield or simplify the production process are warranted. In this context, the exosome-associated AAV vectors (exo-AAVs) are reported to be therapeutically very efficient during ocular gene transfer15 and in HCC.16 They are also known to be resistant to neutralizing antibodies in comparison to conventionally purified AAV vectors17,18 and thus could have significant therapeutic potential.
Several studies have utilized AAV vectors containing suicide genes for cancer therapy.12,19−21 A nonimmunogenic iCasp9 system has also been explored for suicide gene therapy of cancers, including HCC.22 This analogue of Caspase 9 is derived from the mammalian Caspase 9 fused to a human FK506 binding protein (FKBP), allowing conditional dimerization to AP20187, a small, bioinert molecule.22 While we have previously found that the AAV-iCasp9 system is effective in murine models of HCC16,19,22 however, concerns related to off-target effects that could result from using a ubiquitous promoter such as cytomegalovirus (CMV) immediate early enhancer and chicken β actin promoter (CAG) driving the suicide gene remain. Thus, the development and directed expression of the iCasp9 suicide gene, specifically within the HCC cells, is desirable for its clinical translation. We utilized the AAV6 serotype to improve phenotypic rescue in HCC as it has been reported to target liver cells efficiently, including Huh7 cells.23,24
One of the most practical strategies to direct and limit the expression of the suicide gene product to a specific tissue, such as the liver, is to engineer the regulatory elements in the transgene-containing vectors. Previous studies have highlighted the potential of the LP1 promoter (consisting of core liver-specific elements from the human apolipoprotein hepatic control region) for liver-directed human factor IX expression.25,26 This LP1 promoter has so far not been tested for delivering a suicide gene in the context of HCC. Similarly, baculoviral IAP repeat-containing 5 (BIRC5), also known as “Survivin”, is one of the major drivers of the development and progression of multiple cancers, including HCC.27,28 The BIRC5 gene promoter is highly tumor-specific,29,30 thus presenting the possibility of its inclusion as a promoter driving the suicide gene. Importantly, the functional promoter region of the BIRC5 gene spans 1456 bp,31 but a shorter fragment of 269 bp core promoter is reported to have necessary promoter activity and specificity to drive target gene expression in cancer cells and not in healthy noncancerous cells.32 The inclusion of other cancer-specific regulatory elements in the transgene-containing vectors, such as cancer-specific transcription factors, may also be beneficial. One such element is E2F1, a transcription activator that recognizes and interacts with DNA through dimerization proteins via the E2 recognition site, 5′-TTTC[CG]CGC-3′.33,34 E2F1 is also a cancer biomarker and has been shown to be associated with a number of cancers, including HCC.35,36 Hence, together with a cancer-specific gene (BIRC5) promoter, it can help in fine-tuning the suicide gene expression within the cancer cells.
In the present study, we evaluated these targeted promoter systems for their efficacy, with the aim of increasing the therapeutic index and limiting the off-target toxicity of the naked AAV and exo-AAV vectors both in vitro and in vivo.
Results
Development of Liver-Specific LP1 and Cancer-Specific BIRC5 Promoter-Containing iCasp9 Vectors
For the development of promoter-optimized AAV-iCasp9 vectors, a hybrid CAG promoter composed of the CMV immediate early enhancer and chicken β actin promoter from the pAAV-CAG-iCasp9 (donor plasmid) was used, and the promoter was replaced with either an LP1 (along with Kozak) or BIRC5 promoter (along with E2F1 binding site). The developed vectors were validated by Sanger sequencing (GenScript, New Jersey). A schematic of the linear plasmid map is presented in Figure 1. The size of the plasmid obtained after cloning was ∼6.1 and ∼6.0 kb for pAAV-LP1Kozak-iCasp9 and pAAV-E2F1Birc5Kozak-iCasp9, respectively.
Figure 1.
Schematic representation of the cloning strategy for the development of targeted vectors. The CAG promoter from the donor plasmid (pAAV-CAG-iCasp922) was replaced with an LP1 promoter. A consensus Kozak sequence (GCCGCCACC) was added before the iCasp9 transgene to generate the pAAV-LP1Kozak-iCasp9 construct. For the second plasmid, the LP1 promoter was replaced by six repeats of the E2F1 binding site (6X E2F1) and a BIRC5 promoter. Cloning resulted in two plasmids, namely, pAAV-LP1Kozak-iCasp9 and pAAV-E2F1Birc5Kozak-iCasp9.
AAV6-iCasp9 Vectors and Exo-AAV6-iCasp9 Exhibit Efficient Cytotoxicity In Vitro
We further corroborated the novel constructs in human hepatocellular carcinoma cell line (Huh7) after packaging AAV6-CAG-iCasp9, AAV6-LP1Kozak-iCasp9, and AAV6-E2F1Birc5Kozak-iCasp9. Our data demonstrates that all three vectors caused significant cell death in Huh7 cells. Cells infected with AAV6-CAG-iCasp9 vectors showed 78% cell viability, while those infected with AAV6-E2F1Birc5Kozak-iCasp9 (54%) and AAV6-LP1Kozak-iCasp9 (51%) vectors had reduced cell viability as well (Figure 2A). The survival of Huh7 cells between the AAV6-E2F1Birc5Kozak-iCasp9 and AAV6-LP1Kozak-iCasp9 vector-infected groups was comparable. When treated with Exo-AAV6-CAG-iCasp9, Exo-AAV6-LP1Kozak-iCasp9, and Exo-AAV6-E2F1Birc5Kozak-iCasp9 vectors, the Huh7 cell viability decreased to 70.31%, 61.17%, and 60.46%, respectively (Figure 2B). In both AAV6-iCasp9 and exo-AAV6-iCasp9 versions, AAV6-LP1Kozak-iCasp9 and AAV6-E2F1Birc5Kozak-iCasp9 exhibited a significant (p ≤ 0.05) decrease in cell survival when compared to AAV6-CAG-iCasp9 vector-infected cells.
Figure 2.
Cytotoxic effect of targeted AAV-iCasp9 and exo-AAV6-iCasp9 vectors in vitro. The viability of Huh7 cells 72 h after transduction was determined using an ATP assay. Data are represented in comparison to mock-treated cells. Cell viability graph for (A) AAV-iCasp9 vectors and (B) exo-AAV6-iCasp9 vectors. Cell control: mock-treated; positive control: 0.1% Triton X-100. Data are presented as mean ± SD (for each condition, n = 3 replicates, **p ≤ 0.01, ***p ≤ 0.001 in comparison to cell control, #p ≤ 0.05 in comparison to AAV6-CAG-iCasp9 or Exo-AAV6-CAG-iCasp9).
Tumor Growth Is Substantially Inhibited with Targeted Suicide Gene Vectors In Vivo
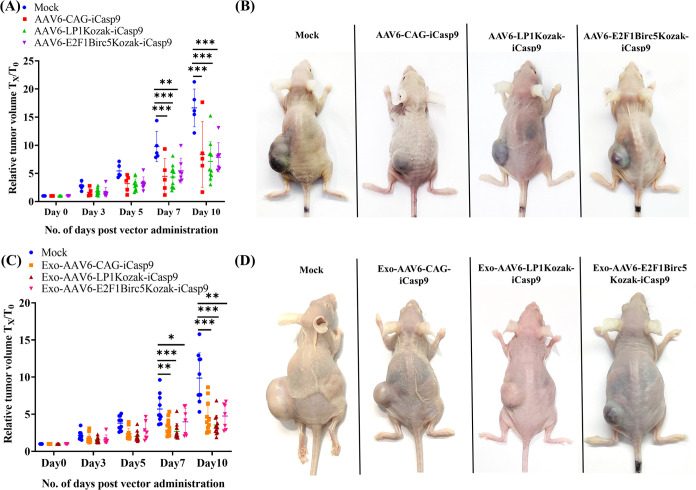
To confirm the therapeutic utility of AAV6-iCasp9 vectors and their exosomal versions, we tested them in a xenotransplantation model of HCC, as described earlier.22 Briefly, athymic nude mice were transplanted with ∼5 million Huh7 cells in a mixture of 25% Matrigel to develop ectopic tumors of 100–150 mm3 in size. These animals were either mock (PBS) administered or administered with 1 × 1010 vg of AAV6 vectors carrying inducible Caspase 9 intratumorally. Our data provided in Figure 3 show a significant regression in the tumor volume 7 days after treatment in all 3 treated groups: AAV6-CAG-iCasp9 (∼[approximately] 2.19 fold; p ≤ 0.001), AAV6-LP1Kozak-iCasp9 (∼2.24 fold; p ≤ 0.001), and AAV6-E2F1Birc5Kozak-iCasp9 (∼1.78 fold; p ≤ 0.01) vectors when compared to animals that were mock-treated (Figure 3A,B). At day 10, a similar level of phenotypic rescue was observed in the treated groups AAV6-CAG-iCasp9 (∼1.99 fold; p ≤ 0.001), AAV6-LP1Kozak-iCasp9 (∼2.33 fold; p ≤ 0.001), and AAV6-E2F1Birc5Kozak-iCasp9 (∼2.11 fold; p ≤ 0.001) vectors when compared to mock-treated animals. Similar data were obtained with Exo-AAV6-CAG-iCasp9 (∼1.96 fold; p ≤ 0.01 at day 7 and ∼2.30 fold; p ≤ 0.001 at day 10), Exo-AAV6-LP1Kozak-iCasp9 (∼2.27 fold; p ≤ 0.001 at day 7 and ∼2.86 fold; p ≤ 0.001 at day 10), and Exo-AAV6-E2F1Birc5Kozak-iCasp9 (∼1.60 fold; p ≤ 0.05 at day 7 and ∼2.13 fold; p ≤ 0.01 at day 10) vectors when compared to animals that were mock-treated (Figure 3C,D). The iCasp9 transgene in combination with AP20187 resulted in tumor regression, as we have recently reported in another study that the vector alone lacks the ability to bring about cell death.37 These data highlight that the LP1 or E2F1-Birc5 promoter is similar in efficacy in comparison to that of the robust CAG promoter system.
Figure 3.
Therapeutic effect of AAV6-iCasp9 vectors and their exosome counterparts in an HCC xenograft model. Graphical representation of tumor regression in vivo after suicide gene therapy with naked AAV6-iCasp9 vectors (A) and Exo-AAV6-iCasp9 vectors (C). Representative images of test animals from different experimental groups 10 days post vector administration with AAV6-iCasp9 vectors (B) and Exo-AAV6-iCasp9 vectors (D). The treated groups AAV6-CAG-iCasp9 (n = 5), AAV6-LP1Kozak-iCasp9 (n = 11), and AAV6-E2F1Birc5Kozak-iCasp9 (n = 7) demonstrated significant tumor regression in comparison to mock-injected animals (n = 5). A similar pattern of tumor regression was also found in animals that received Exo-AAV6-CAG-iCasp9 (n = 11), Exo-AAV6-LP1Kozak-iCasp9 (n = 12), and Exo-AAV6-E2F1Birc5Kozak-iCasp9 (n = 7) vectors when compared to mock-injected animals (n = 10). Two-way ANOVA was performed for statistical analysis. Animals that died or had necrotic tumors were excluded from the analysis. Data are presented as mean ± SD, *p ≤ 0.05; **p ≤ 0.01; and ***p ≤ 0.001 in comparison to mock.
Detection of Apoptosis in Tumor Tissues after Suicide Gene Transfer
Treated animals were humanely euthanized, and tumors were isolated 10 days after gene therapy. Hematoxylin and eosin staining of tumor tissue sections was performed for morphological evaluation. The mock-treated tissue samples exhibited a higher number of mitotically active and large proliferating cells (Figure 4A,E). Histological analysis of the vector-treated groups depicted apoptotic cells, which presented with well-defined features, including cell shrinkage, condensation, and deep eosinophilia of the cytoplasm along with a pyknotic, round to irregular nucleus, as described earlier.38 The AAV6-iCasp9- and exo-AAV6-iCasp9-treated groups demonstrated a high number of apoptotic cells with pyknotic nuclei (Figure 4B–D,F–H).
Figure 4.
Morphological and molecular characterization of apoptotic cells in tumor tissue. Hematoxylin and eosin staining was performed on 10 μm sections of a paraffin-embedded tumor tissue, and microscopic examination was conducted using a Leica DMi8 (Leica microsystems, Wetzlar, Germany). (A–H) 100× images, Scale bar: 25 μm. Red arrows indicate mitotic and proliferating cells and white arrows indicate pyknotic, dense, or fragmented nuclei. TUNEL staining of tumor sections was performed, and confocal imaging was done (I, K) using an LSM780NLO Carl Zeiss GmbH (Carl Zeiss AG, Jena, Germany), 63× images, Scale bar: 50 μm. Quantification of TUNEL-positive cells in tumor sections using ImageJ software (J, L) is shown. Data are presented as mean ± SD, *p ≤ 0.05; **p ≤ 0.01; and ***p ≤ 0.001 in comparison to mock.
To determine the impact of iCasp9-based gene therapy at the molecular level, a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed. The imaging data from the tumor tissue from all three treated groups AAV6-CAG-iCasp9, AAV6-LP1Kozak-iCasp9, and AAV6-E2F1Birc5Kozak-iCasp9 and respective exosome counterparts showed a higher number of TUNEL-positive cells (red). This signifies massive cell death in the treated groups, as compared to the control group (Figure 4I,K). We further quantified the fluorescence signal from the TUNEL-positive cells, which revealed that all three treated groups AAV6-CAG-iCasp9, AAV6-LP1Kozak-iCasp9, and AAV6-E2F1Birc5Kozak-iCasp9 and the corresponding exo-AAVs had significantly higher integrated density per unit area of TUNEL positivity when compared to the mock group (Figure 4J,L).
Discussion
The preferred course of treatment for HCC patients with early stage illness and poor liver function is surgical resection. However, despite recent advancements, hepatectomy remains linked to an increased rate of morbidity and mortality.39,40 To preserve liver function, it is crucial to develop a strategy that exclusively targets HCC without harming normal cells. In our previous study, we utilized an AAV serotype 2-based vector to test the potency of iCasp9 to induce cytotoxicity, where the transgene was under the influence of a strong ubiquitous promoter (CAG).22 In the present study, we reasoned that the use of a liver-specific (LP1) or cancer-specific promoter (BIRC5), with additional molecular features such as Kozak and cancer-specific transcription factors, could be a better approach than using a ubiquitous promoter to limit off-target toxicity.
AAV6 vectors have been used in cancer therapies to deliver transgenes for therapeutic effect. In an earlier study, we utilized AAV6 to deliver a CAG promoter-driven iCasp9 transgene for the therapeutic rescue of HCC and observed significant tumor regression in a mouse xenograft model.22 By employing LP1 and BIRC5, we made an effort to make iCasp9 expression more targetable. We additionally incorporated a consensus Kozak motif for improved protein production into the vectors. Most eukaryotic cells use the Kozak sequence as a translation initiation site, and it has been observed that mutations in this area can alter the translation status and cause disease.41 A Kozak consensus sequence has therefore been included in our study to boost translation of the coding region. This Kozak sequence has already been identified as a consensus sequence in 699 vertebrate mRNAs for initiation of translation.42
Specific targeting of liver/cancer cells is crucial in the treatment of HCC. Although the LP1 promoter has not been explored in this context previously, it has been used to drive coagulation factor IX expression to the liver25,26 in the case of hemophilia B gene therapy. BIRC5 has been widely studied, and a recent study demonstrated that the herpes simplex virus thymidine kinase (HSV-TK) suicide gene system, when controlled via a survivin promoter, selectively inhibited HCC cell proliferation in a xenograft mice model by enhancing apoptosis, thereby increasing survival.43 We also incorporated an E2F1 transcription factor binding site to elevate iCasp9 expression. E2F1 is one of the members of the E2F family that often operates as a transcriptional activator for genes involved in cell cycle progression and nucleotide synthesis. The E2F1 promoter within an adenoviral vector has been used to demonstrate that a transgene driven by this promoter can cause tumor-selective gene expression and further eradicate established gliomas in vivo.(44) Taking a cue from these studies, we demonstrated the therapeutic effect of the iCasp9 system when delivered with tissue/cancer-specific promoters using naked AAV6 vectors and their exosomal versions. The addition of the exosomal AAV fraction, which is otherwise discarded during routine recombinant vector packaging, is likely to reduce the cost associated with suicide gene therapy. Our results also demonstrate that the efficacy of the tissue-specific (LP1) and cancer-specific (BIRC5) promoter is similar to the highly robust CAG promoter system either in AAV or exo-AAV systems. Several studies have shown superior transgene expression with a strong CAG promoter system over liver-directed or cancer-specific promoters.45,46 Although tissue-specific promoters are generally considered weak in nature when compared to ubiquitous promoters,47 we observed that the phenotypic outcome with the LP1 or BIRC5 promoters employed here was similar to a strong CAG ubiquitous promoter in vivo. Similar observations have been made in the context of neuron-specific promoters that resulted in comparable green fluorescent protein (GFP) expression in the brain to the CAG promoter.48 In our previous reports, we have utilized higher doses of vector where AAV2-iCasp9 and exo-AAV6-iCasp9 vectors were administered at a dose of 5 × 1010 vgs/animal22 and 2 × 1010 vgs/animal,16 respectively. Hence, in the current study, we tested a dose of 1 × 1010 vgs/animal to determine if phenotypic rescue is maintained at a lower dose. The observation of similar but specific cytotoxicity with the LP1 and E2F1/BIRC5 vectors, even at a 5-fold lower dose of vectors administered (1 × 1010 vgs vs 5 × 1010 vgs/animal) compared to the previous study,22 augurs well for their potential use in humans.
Our study is proof-of-concept for the potential of targeted AAV6 vectors, whose outcomes we believe can be improved further. From our earlier studies,16,22 we have innovated to generate different promoter systems (LP1 and BIRC5) in AAV6 serotype that demonstrate their potential for HCC treatment. However, in the absence of complete tumor ablation, further clinical use will be contingent upon their evaluation in combination with Sorafenib, the current standard of care, or novel chemotherapeutic agents as a chemogene therapy strategy to achieve maximum remission.
Materials and Methods
Design and Synthesis of Modified Inducible Caspase 9 Constructs
The iCasp9 transgene was cloned into the AAV backbone along with LP1 (liver-specific) and BIRC5 (cancer-specific) promoters. The plasmid pAAV-CAG-iCasp9 was used as a donor plasmid, and the LP125 promoter was added in place of the CAG promoter along with a Kozak sequence (GCCGCCACC)49 upstream of the iCasp9 transgene to yield pAAV-LP1Kozak-iCasp9. The LP1 promoter was replaced with the BIRC532 promoter to generate the pAAV-Birc5Kozak-iCasp9 vectors. To improve cancer cell targeting, six tandem repeats of the E2F1 factor binding site, 5′-TTTC[CG]CGC-3′,33 were incorporated upstream of the BIRC5 promoter to yield pAAV-E2F1Birc5Kozak-iCasp9. The cloning and synthesis of these vectors were performed using a PCR based method named CloneEZ, and Sanger sequencing was performed for confirmation of cloning (Genscript).
Cell Lines and Reagents
The AAV packaging cell line (AAV293) was procured from Stratagene (San Diego, CA). The human hepatocellular carcinoma (Huh7) cell line was a kind gift from S. Das (Indian Institute of Science, Bengaluru). Ciprofloxacin was purchased from HiMedia Laboratories, Mumbai, India, and piperacillin from MP Biomedicals, Irvin, CA. Iscove’s modified Dulbecco’s medium (IMDM) and fetal bovine serum (FBS) were procured from Gibco (Life Technologies, Carlsbad, CA). Cells were cultured at 37 °C with 5% CO2 and maintained in IMDM with 10% FBS, 10 μg/mL ciprofloxacin and piperacillin each, and sodium bicarbonate (Sigma-Aldrich, St Louis, MO).
Viral Vector Packaging and Quantification
The packaging cell line AAV293 was expanded to 40 Petri dishes (150 mm3). At a 70–80% confluence, cells were transfected using three plasmids: AAV-rep/cap (pAAV6WT), an adenoviral helper plasmid (pHelper) with three separate transgenes pAAV-CAG-iCasp9, pAAV-LP1Kozak-iCasp9, or pAAV-E2F1Birc5Kozak-iCasp9 in the presence of 0.1% polyethylenimine (PEI; Polysciences Inc., Taipei, Taiwan) as a transfecting reagent. AAV genome titers were estimated using a quantitative real-time polymerase chain reaction (qPCR).50 Titers were generated from two replicate analyses and calculated as average vector genomes per milliliter (vgs/mL).
Exosome Isolation
Exosomes were harvested from the supernatant media collected 68–72 h post-transfection of AAV293 cells and purified using density gradient centrifugation, as described previously.16 The supernatant media were centrifuged at 3000 rpm for 15 min for cell sedimentation, followed by 20,000g for 1 h to remove cell debris; further, centrifugation of the same media at 100,000g for 1.5 h yielded a pellet, which was resuspended in 200 μL of phosphate-buffered saline (PBS). Titers were estimated using qPCR and calculated as average vgs/mL.
Cell Cytotoxicity Assay
The viability was tested in Huh7, seeded at a cell density of 1.5 × 104 cells/well in a 48-well plate. Cells were transduced at a multiplicity of infection (MOI) of 10,000 with AAV6-CAG-iCasp9, AAV6-LP1Kozak-iCasp9, and AAV6-E2F1Birc5Kozak-iCasp9 and their respective Exo-AAV vectors. The dimerizer drug AP20187 (50 nM; Ariad Pharmaceuticals, Cambridge, MA) was added 24 h post-transduction, and cells were incubated for 48 h at 37 °C.22 Percentage cell viability was measured using an ATP assay kit as per the manufacturer’s protocol (CellTiter-Glo, Promega, Madison, WI).
Suicide Gene Therapy in a Xenograft Mouse Model of HCC
Athymic nude mice (8 to 10 weeks old) were used for the study. The animal experiments were approved by the Institutional Animal Ethics Committee (IIT Kanpur, India). The xenograft model was developed by injection of ∼5 million Huh7 cells51 along with 25% ECM (extracellular matrix) gel (Sigma-Aldrich, St Louis, MO), subcutaneously. Animals were randomly divided into 4 groups: mock (n = 5), AAV6-CAG-iCasp9 (n = 5), AAV6-LP1Kozak-iCasp9 (n = 11), and AAV6-E2F1Birc5Kozak-iCasp9 (n = 7) after they developed tumors (100–150 mm3).52 With exosomal AAV vectors, the treatment groups were mock (n = 10), Exo-AAV6-CAG-iCasp9 (n = 11), Exo-AAV6-LP1Kozak-iCasp9 (n = 12), and Exo-AAV6-E2F1Birc5Kozak-iCasp9 (n = 7). Animals in the mock group received an equal volume of PBS. A dose of 1 × 1010 vgs of the vector was administered intratumorally, and dimerizer drug AP20187 (1 mg/kg body weight) was injected intraperitoneally, thrice at 48 h intervals. After 10 days of vector administration, the animals were humanely euthanized, and the tumor was collected. The tumor volume was calculated using the formula V = (L × W2)/2, where L and W are the longest and smallest diameters (mm), respectively. The relative tumor volume (RTV) in the treated and control mice was calculated as reported earlier, RTV = Vt/V0, where Vt is the volume on each day and V0 is the tumor volume at the beginning of the treatment.53
Histological Analysis
Tumors from the test groups were harvested and fixed in 10% buffered formalin overnight. After paraffin embedding, 10 μm sections were prepared. Hematoxylin and eosin staining was performed, and apoptotic bodies in the tumor tissue were identified using Leica DMi8 (Leica microsystems, Wetzlar, Germany).
In Situ TUNEL Assay
The tumor tissue was fixed in 10% buffered formalin. The samples were embedded in paraffin and sectioned into 10 μm slices using a microtome (Leica, Wetzlar, Germany). The TUNEL assay was performed to identify apoptotic cells according to standard protocols (Roche, Basel, Switzerland). Sections were also counterstained with DAPI (Thermo Fischer, Waltham, MA) and mounted using FluorSave (Sigma-Aldrich). Images were acquired by confocal imaging using an LSM780NLO Carl Zeiss GmbH (Carl Zeiss AG, Jena, Germany). A minimum of three tissue sections per tumor (2 tumors per group) were analyzed for quantification of TUNEL-positive cells, which was performed using ImageJ software.
Statistical Analysis
Statistical analysis was performed using either unpaired two-tailed Student’s t test or by one-way/two-way analysis of variance test (ANOVA), as applicable. A p value of ≤0.05 was considered statistically significant. All analyses were performed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA).
Acknowledgments
The authors thank the central experimental animal facility, IIT Kanpur, for housing the animals. They also thank Dr. Saraswat Pathology, Kanpur, for the preparation of paraffin sections and hematoxylin and eosin staining of tumor samples. Images were partially created using BioRender.com.
Author Contributions
V.S. conducted the experiments. S.P. and N.K. contributed to in vivo administrations. G.R.J. conceived, designed, and supervised the study. V.S. and G.R.J. wrote the manuscript.
This work was supported through intramural grants of IIT Kanpur to G.R.J.
The authors declare the following competing financial interest(s): The authors declare that IIT Kanpur has filed patent applications on AAV vectors.
Notes
Ethical Approval Statement: The animal studies were approved by the Institutional Animal Ethics Committee (IIT Kanpur, India).
References
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2021, 71 (3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Daher S.; Massarwa M.; Benson A. A.; Khoury T. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J. Clinic. Transl. Hepatol. 2018, 6 (1), 69. 10.14218/JCTH.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y.-H.; Lu S.-N.; Chen C.-L.; Cheng Y.-F.; Lin C.-Y.; Hung C.-H.; Chen C.-H.; Changchien C.-S.; Hsu H.-C.; Hu T.-H.; et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur. J. Cancer 2010, 46 (4), 744–751. 10.1016/j.ejca.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Sun J. Y.; Yin T.; Zhang X. Y.; Lu X. J. Therapeutic advances for patients with intermediate hepatocellular carcinoma. J. Cell. Physiol. 2019, 234 (8), 12116–12121. 10.1002/jcp.28019. [DOI] [PubMed] [Google Scholar]
- Gurzu S.; Kobori L.; Fodor D.; Jung I. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. BioMed. Res. Int. 2019, 2019, 2962580. 10.1155/2019/2962580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-C.; Chen C.-F.; Ho C.-T.; Liu J.-J.; Liu T.-Z.; Chern C.-L. γ-Glutamylcysteine synthetase (γ-GCS) as a target for overcoming chemo-and radio-resistance of human hepatocellular carcinoma cells. Life Sci. 2018, 198, 25–31. 10.1016/j.lfs.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Liu M.; Hu Q.; Tu M.; Wang X.; Yang Z.; Yang G.; Luo R. MCM6 promotes metastasis of hepatocellular carcinoma via MEK/ERK pathway and serves as a novel serum biomarker for early recurrence. J. Experiment. Clin. Cancer Res. 2018, 37 (1), 1–13. 10.1186/s13046-017-0669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.-L.; Shen M.-N.; Hu B.; Wang B.-L.; Yang W.-J.; Lv L.-H.; Wang H.; Zhou Y.; Jin A.-L.; Sun Y.-F. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J. Hematol. Oncol. 2019, 12 (1), 1–17. 10.1186/s13045-019-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J.; Lau E. Y. T.; Ching R. H. H.; Cheng B. Y. L.; Ma M. K. F.; Ng I. O. L.; Lee T. K. W. Nuclear factor kappa B–mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015, 62 (2), 534–545. 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- Giannini E. G.; Farinati F.; Ciccarese F.; Pecorelli A.; Rapaccini G. L.; Di Marco M.; Benvegnù L.; Caturelli E.; Zoli M.; Borzio F.; et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015, 61 (1), 184–190. 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- Mohammadinejad R.; Dehshahri A.; Sagar Madamsetty V.; Madamsetty V. S.; Zahmatkeshan M.; Tavakol S.; Makvandi P.; Khorsandi D.; Pardakhty A.; Ashrafizadeh M.; Ghasemipour Afshar E.; Afshar E. G. In vivo gene delivery mediated by non-viral vectors for cancer therapy. J. Controlled Release 2020, 325, 249–275. 10.1016/j.jconrel.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.; Khan N.; Jayandharan G. R. Vector engineering, strategies and targets in cancer gene therapy. Cancer Gene Ther. 2022, 29 (5), 402–417. 10.1038/s41417-021-00331-7. [DOI] [PubMed] [Google Scholar]
- Luo J.; Luo Y.; Sun J.; Zhou Y.; Zhang Y.; Yang X. Adeno-associated virus-mediated cancer gene therapy: current status. Cancer Lett. 2015, 356 (2), 347–356. 10.1016/j.canlet.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016, 21, 75–80. 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya S.; Jayandharan G. R. Exosome-associated SUMOylation mutant AAV demonstrates improved ocular gene transfer efficiency in vivo. Virus Res. 2020, 283, 197966 10.1016/j.virusres.2020.197966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.; Maurya S.; Bammidi S.; Jayandharan G. R. AAV6 vexosomes mediate robust suicide gene delivery in a murine model of hepatocellular carcinoma. Mol. Ther. Methods Clin. Dev. 2020, 17, 497–504. 10.1016/j.omtm.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B.; Fitzpatrick Z.; Crommentuijn M. H.; Mu D.; Maguire C. A. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 2014, 35 (26), 7598–7609. 10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliani A.; Boisgerault F.; Fitzpatrick Z.; Marmier S.; Leborgne C.; Collaud F.; Simon Sola M.; Charles S.; Ronzitti G.; Vignaud A.; et al. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 2017, 1 (23), 2019–2031. 10.1182/bloodadvances.2017010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.; Cheemadan S.; Saxena H.; Bammidi S.; Jayandharan G. R. MicroRNA-based recombinant AAV vector assembly improves efficiency of suicide gene transfer in a murine model of lymphoma. Cancer Medicine 2020, 9 (9), 3188–3201. 10.1002/cam4.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U. T.; Bentler M.; Kaniowska D.; Morgan M.; Büning H. Towards clinical implementation of adeno-associated virus (AAV) vectors for cancer gene therapy: current status and future perspectives. Cancers 2020, 12 (7), 1889. 10.3390/cancers12071889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian F.; Ye Q.; Feng B.; Cheng H.; Niu S.; Fan N.; Wang D.; Wang Z. rAAV9-UPII-TK-EGFP can precisely transduce a suicide gene and inhibit the growth of bladder tumors. Cancer Biol. Therapy 2020, 21 (12), 1171–1178. 10.1080/15384047.2020.1844115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.; Bammidi S.; Chattopadhyay S.; Jayandharan G. R. Combination suicide gene delivery with an adeno-associated virus vector encoding inducible caspase-9 and a chemical inducer of dimerization is effective in a xenotransplantation model of hepatocellular carcinoma. Bioconjugate Chem. 2019, 30 (6), 1754–1762. 10.1021/acs.bioconjchem.9b00291. [DOI] [PubMed] [Google Scholar]
- Rezvani M.; Español-Suñer R.; Malato Y.; Dumont L.; Grimm A. A.; Kienle E.; Bindman J. G.; Wiedtke E.; Hsu B. Y.; Naqvi S. J.; et al. In vivo hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell 2016, 18 (6), 809–816. 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayroo R.; Nolasco D.; Yin Z.; Colon-Cortes Y.; Pandya M.; Ling C.; Aslanidi G. Development of novel AAV serotype 6 based vectors with selective tropism for human cancer cells. Gene Ther. 2016, 23 (1), 18–25. 10.1038/gt.2015.89. [DOI] [PubMed] [Google Scholar]
- Nathwani A. C.; Tuddenham E. G.; Rangarajan S.; Rosales C.; McIntosh J.; Linch D. C.; Chowdary P.; Riddell A.; Pie A. J.; Harrington C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365 (25), 2357–2365. 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary B.; Maurya S.; Kumar M.; Bammidi S.; Kumar V.; Jayandharan G. R. Molecular engineering of adeno-associated virus capsid improves its therapeutic gene transfer in murine models of hemophilia and retinal degeneration. Mol. Pharmaceutics 2019, 16 (11), 4738–4750. 10.1021/acs.molpharmaceut.9b00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiris I.; Nastos K.; Karakatsanis A.; Theodosopoulos T.; Karandrea D.; Kondi Pafiti A.; Contis J. Survivin expression in hepatocellular carcinoma. Correlation with clinicopathological characteristics and overall survival. J. BUON 2019, 24 (5), 1934–1942. [PubMed] [Google Scholar]
- Yu J.; Wang Z.; Zhang H.; Wang Y.; Li D.-Q. Survivin-positive circulating tumor cells as a marker for metastasis of hepatocellular carcinoma. World J. Gastroenterol. 2021, 27 (43), 7546. 10.3748/wjg.v27.i43.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka K.; Spain C.; Yen A.; Overlid N.; Gebremedhin S.; Düzgüneş N. Correlation between the levels of survivin and survivin promoter-driven gene expression in cancer and non-cancer cells. Cellular Molecul. Biol. Lett. 2009, 14 (1), 70–89. 10.2478/s11658-008-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García D.; Manero-Rupérez N.; Quesada R.; Korrodi-Gregório L.; Soto-Cerrato V. Therapeutic strategies involving survivin inhibition in cancer. Med. Res. Rev. 2019, 39 (3), 887–909. 10.1002/med.21547. [DOI] [PubMed] [Google Scholar]
- LI F.; ALTIERI D. C. Transcriptional analysis of human survivin gene expression. Biochem. J. 1999, 344 (2), 305–311. 10.1042/bj3440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Cao Z.; Li F.; Post D. E.; Van Meir E. G.; Zhong H.; Wood W. C. Tumor-specific gene expression using the survivin promoter is further increased by hypoxia. Gene Ther. 2004, 11 (15), 1215–1223. 10.1038/sj.gt.3302280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z.; Lin M.; Li C.; Liu H.; Gong C. J. A. A comprehensive review of the roles of E2F1 in colon cancer. Am. J. Cancer Res. 2020, 10 (3), 757. [PMC free article] [PubMed] [Google Scholar]
- Fang Z.; Wang Y.; Wang Z.; Xu M.; Ren S.; Yang D.; Hong M.; Xie W. ERINA Is an Estrogen-Responsive LncRNA That Drives Breast Cancer through the E2F1/RB1 PathwayLncRNA ERINA Play an Oncogenic Role in Breast Cancer. Cancer Res. 2020, 80 (20), 4399–4413. 10.1158/0008-5472.CAN-20-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Chen J.; Zhao L.; Song W.; Xuan Z.; Chen J.; Li Z.; Song G.; Hong L.; Song P.; Zheng S. CDCA5, transcribed by E2F1, promotes oncogenesis by enhancing cell proliferation and inhibiting apoptosis via the AKT pathway in hepatocellular carcinoma. J. Cancer 2019, 10 (8), 1846. 10.7150/jca.28809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanelle J.; Stiewe T.; Theseling C. C.; Peter M.; Pützer B. M. Gene expression changes in response to E2F1 activation. Nucleic Acids Res. 2002, 30 (8), 1859–1867. 10.1093/nar/30.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S.; Singh V.; Kumar N.; Jayandharan G. R. Inducible caspase 9-mediated suicide gene therapy using AAV6 vectors in a murine model of breast cancer. Mol. Ther.--Methods Clin. Dev. 2023, 31, 101166. 10.1016/j.omtm.2023.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. A.; Dixon D.; Hailey J. R.; Harada T.; Herbert R. A.; Maronpot R. R.; Nolte T.; Rehg J. E.; Rittinghausen S.; Rosol T. J.; et al. Recommendations from the INHAND apoptosis/necrosis working group. Toxicol. Pathol. 2016, 44 (2), 173–188. 10.1177/0192623315625859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei A. C.; Tung-Ping Poon R.; Fan S.; Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. J. British Surgery 2003, 90 (1), 33–41. 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- Xourafas D.; Pawlik T. M.; Cloyd J. M. Early morbidity and mortality after minimally invasive liver resection for hepatocellular carcinoma: a propensity-score matched comparison with open resection. J. Gastrointestinal Surgery 2019, 23 (7), 1435–1442. 10.1007/s11605-018-4016-2. [DOI] [PubMed] [Google Scholar]
- Mohan R. A.; van Engelen K.; Stefanovic S.; Barnett P.; Ilgun A.; Baars M. J.; Bouma B. J.; Mulder B. J.; Christoffels V. M. Mutation in the Kozak sequence of GATA4 hampers translation in a family with atrial septal defects. Am. J. Med. Genetics Part A 2014, 164 (11), 2732–2738. 10.1002/ajmg.a.36703. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987, 15 (20), 8125–8148. 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X.; Wang X.; Wei Y.; Wen C.; Zhang Q.; Xu C.; Liu C.; Zhang C.; Meng F.; Zhao N.; et al. ApoE-modified liposomes mediate the antitumour effect of survivin promoter-driven HSVtk in hepatocellular carcinoma. Cancer Gene Ther. 2020, 27 (10), 754–767. 10.1038/s41417-019-0145-3. [DOI] [PubMed] [Google Scholar]
- Parr M. J.; Manome Y.; Tanaka T.; Wen P.; Kufe D. W.; Kaelin W. G. Jr; Fine H. A. Tumor-selective transgene expression in vivo mediated by an E2F-responsive adenoviral vector. Nat. Med. 1997, 3 (10), 1145–1149. 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- Kang J.; Huang L.; Zheng W.; Luo J.; Zhang X.; Song Y.; Liu A. Promoter CAG is more efficient than hepatocyte-targeting TBG for transgene expression via rAAV8 in liver tissues. Molecular Med. Rep. 2022, 25 (1), 16. 10.3892/mmr.2021.12532. [DOI] [PubMed] [Google Scholar]
- Sato Y.; Tanaka K.; Lee G.; Kanegae Y.; Sakai Y.; Kaneko S.; Nakabayashi H.; Tamaoki T.; Saito I. Enhanced and specific gene expression via tissue-specific production of Cre recombinase using adenovirus vector. Biochem. Biophys. Res. Commun. 1998, 244 (2), 455–462. 10.1006/bbrc.1997.8087. [DOI] [PubMed] [Google Scholar]
- Nakamura S.; Watanabe S.; Ohtsuka M.; Maehara T.; Ishihara M.; Yokomine T.; Sato M. Cre-loxP system as a versatile tool for conferring increased levels of tissue-specific gene expression from a weak promoter. Mol. Reprod. Dev. 2008, 75 (6), 1085–1093. 10.1002/mrd.20847. [DOI] [PubMed] [Google Scholar]
- Finneran D. J.; Njoku I. P.; Flores-Pazarin D.; Ranabothu M. R.; Nash K. R.; Morgan D.; Gordon M. N. Toward Development of Neuron Specific Transduction After Systemic Delivery of Viral Vectors. Front. Neurol. 2021, 12, 685802 10.3389/fneur.2021.685802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P.; Recino A.; Foale R. D.; Zhao J.; Gan S. U.; Wallberg M.; Calne R.; Lever A. M. Promoter optimization of lentiviral vectors for efficient insulin gene expression in canine mesenchymal stromal cells: potential surrogate beta cells. J. Gene Med. 2016, 18 (10), 312–321. 10.1002/jgm.2900. [DOI] [PubMed] [Google Scholar]
- Aurnhammer C.; Haase M.; Muether N.; Hausl M.; Rauschhuber C.; Huber I.; Nitschko H.; Busch U.; Sing A.; Ehrhardt A.; Baiker A. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther: Methods 2012, 23 (1), 18–28. 10.1089/hgtb.2011.034. [DOI] [PubMed] [Google Scholar]
- Hong S. W.; Jung K. H.; Lee H. S.; Choi M. J.; Son M. K.; Zheng H. M.; Hong S. S. SB 365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI 3 K/A kt/mTOR signaling pathway. Cancer Sci. 2012, 103 (11), 1929–1937. 10.1111/j.1349-7006.2012.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H.; Zheng Y.-f.; Ge W.; Wang S.-b.; Mou X.-z. Synergistic anti-tumour effects of quercetin and oncolytic adenovirus expressing TRAIL in human hepatocellular carcinoma. Sci. Rep. 2018, 8 (1), 2182 10.1038/s41598-018-20213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-M.; Kong F.-E.; Li G.-M.; He Y.-T.; Zhang X.-F.; Zhang C.-Y.; Liang J.-K.; Guan X.-Y.; Ma N.-F.; Xie M.-B.; Liu M. Identification and functional characterization of potential oncofetal targets in human hepatocellular carcinoma. STAR Protocols 2022, 3 (4), 101921 10.1016/j.xpro.2022.101921. [DOI] [PMC free article] [PubMed] [Google Scholar]