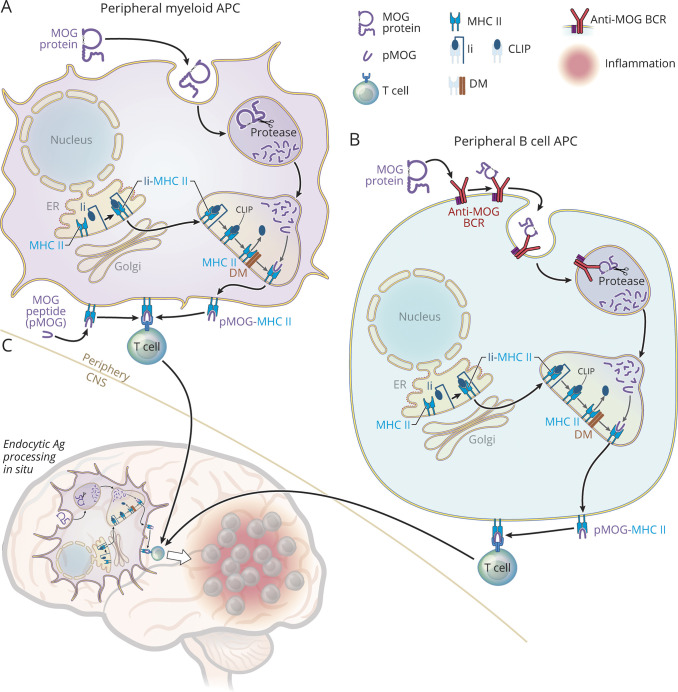
Figure 2. MHC II Endocytic Antigen Processing and Presentation to T Cells in MOG CNS Autoimmunity.
This figure illustrates types of APCs, including myeloid cells (A), B cells (B), and microglia (C) that may participate in MOG CNS autoimmunity. MOG EAE is typically induced by subcutaneous immunization with either MOG protein or MOG peptide (pMOG), which leads to peripheral (outside CNS) activation of pathogenic MOG-specific T cells that traffic into the CNS and initiate CNS inflammation.36 Human MOG protein, which contains proline-42, causes T-B-dependent EAE.36,37 MOG-specific T-cell activation requires recognition of pMOG in association with MHC II molecules expressed on APCs.34 Antigen processing of MOG protein through the MHC II endocytic pathway is required in at least at 2 stages in MOG protein-induced EAE, for initial recognition by MOG-specific T cells in the periphery and for reactivation within the CNS (in situ).34,38 Several molecules, including invariant chain (Ii, CD74), HLA-DM (H-2M) (DM), and proteases participate in MHC II maturation and in orchestrating steps within the endocytic pathway.39 MHC II (α/β) molecules associate with Ii in the endoplasmic reticulum (ER) forming a trimer (Ii-MHC II) and travel through the Golgi to the endosomal compartment. Ii is enzymatically degraded yielding a fragment of Ii, class II Ii peptide (CLIP), which remains bound within the MHC II peptide-binding groove. The MHC II chaperone DM facilitates removal of CLIP, permitting exchange for antigenic peptide (e.g., pMOG). Peripheral myeloid cells capture native antigenic proteins (MOG) (top of A) via phagocytosis or pinocytosis and deliver them to the endolysosomal compartment where they are degraded by proteases into 9–14 amino acid fragments that can bind the MHC II peptide-binding groove.39 Endosomes containing peptide-MHC II complexes fuse with the plasma membrane permitting presentation of peptides (e.g., pMOG-MHC II) to encephalitogenic MOG-specific T cells. In contrast to MOG protein, pMOG immunization (lower left in A) supplies peptide that can bind cell surface MHC II molecules directly, bypassing the need for endocytic processing.34 B cells are exceptionally efficient APCs when they bind antigens (e.g., MOG protein) (top of B) with their B-cell antigen-specific receptors (anti-MOG-BCR) and deliver them to the endolysosomal compartment for processing and association with MHC II molecules.39,40 Independent of whether MOG EAE is induced by immunization with MOG protein, pMOG, or by adoptive transfer of encephalitogenic MOG-specific T cells (not shown), endocytic Ag processing by APCs (e.g., resident microglia) (shown in C) in situ is required for recognition of cognate MOG peptide by MOG-specific T cells that initiate CNS inflammation.34,38 Copyright Xavier Studio, reprinted with permission.