Abstract
Selection of a localization method for nonpalpable breast lesions offers an opportunity for institutions to seek multidisciplinary input to promote value-based, patient-centered care. The diverse range of nonpalpable breast and axillary pathologies identified through increased utilization of screening mammography often necessitates image-guided preoperative localization for accurate lesion identification and excision. Preoperative localization techniques for breast and axillary lesions have evolved to include both wire and nonwire methods, the latter of which include radioactive seeds, radar reflectors, magnetic seeds, and radiofrequency identification tag localizers. There are no statistically significant differences in surgical outcomes when comparing wire and nonwire localization devices. Factors to consider during selection and adoption of image-guided localization systems include physician preference and ease of use, workflow efficiency, and patient satisfaction.
Keywords: wire localization, nonwire localization, multidisciplinary decision making, breast imaging
Introduction
Screening mammography and imaging advancements have resulted in increased detection of nonpalpable breast lesions that require preoperative localization for surgical removal (1). Specifically, screening-detected, early stage breast cancer is often treated with breast-conserving therapy, with the end goals of excising the tumor to negative margins, staging the axilla, and providing satisfactory cosmesis (2). The role of the radiologist is to perform accurate preoperative, image-guided localization of nonpalpable breast and axillary lesions to aid surgical removal. Therefore, multidisciplinary decision making between radiologists and surgeons is necessary to discuss whether breast conservation therapy is appropriate, coordinate the preferred type of preoperative localization, and review surgical specimens to confirm appropriate excision.
Localization has evolved to include wire localization (WL) and nonwire localization (NWL) techniques (Table 1). When comparing surgical outcomes, specifically positive margin rates requiring repeat excision, a single localization technique has not been found to be superior (3). As such, when first selecting or transitioning to a different localization method, institutions should seek multidisciplinary input to select a device that promotes value-based, patient-centered care and ease of clinical use (Table 2). As each localization device has unique benefits and drawbacks, we aim to describe localization workflow and summarize the advantages and disadvantages of both WL and NWL techniques.
Table 1.
Comparison of Wire and Nonwire Localization Devices
| Localization Device | |||||
|---|---|---|---|---|---|
| Wire | I-125 Radioactive Seed | Radar Reflector | Magnetic Seed | Radiofrequency Identification Tag | |
| Graphical representation |
|
|
|

|
|
| Device information | Wire, external placement needle | I-125 radioactive titanium seed, needle delivery system | Infrared-activated electromagnetic wave reflector, needle delivery system | Inducible magnetic device, needle delivery system | Radio wave frequency device, needle delivery system |
| Detector | None | Geiger-Muller survey meter, intraoperative gamma probe | Console | Handheld probe | Handheld disposable probe |
| Device cost, average | $20 | $20–50 | $450 | $400 | $550 |
| Maximum days of implantation | 0–1 days | 14 days | >30 days | >30 days | >30 days |
Table 2.
Multidisciplinary Considerations for Implementation of Wire and Nonwire Localization Device Programs
| Wire Localization (WL) | Nonwire Localization | |
|---|---|---|
| Patient considerations | Fasting for same-day surgery Most economical choice Reported increased anxiety and decreased satisfaction |
Scheduling flexibility Most devices are costly High satisfaction compared to WL |

| ||
| Physician (radiologist and surgeon) considerations | Decades of reliability Path of wire may dictate surgical approach Can be repositioned once deployed Can be placed under MRI guidance Typically placed on the same day as surgery |
No difference in oncologic surgical outcomes when compared to WL Radiologist places device without concern of surgical approach Cannot be repositioned once deployed Radioactive seed is the only device that can be placed under MRI guidance Can be placed on same day or days prior to surgery |
|
| ||
| Institutional considerations | Most economical choice (per device) Surgery and radiology linkage can lead to unexpected costs and operative inefficiencies No multidisciplinary regulatory precautions |
Initial infrastructure acquisition costs in the range of tens of thousands of dollars with ongoing device costs Decouples radiology and surgery schedules, possibly leading to lower operative costs Radioactive seed programs have strict multidisciplinary regulatory precautions |
|
|
Localization Workflow
Localization workflow includes appropriate identification of cases requiring localization, preoperative review of cases with selection of modality and localization technique, image-guided placement of localization devices, surgical excision, and review of the surgical specimen.
When preoperative localization is requested the radiologist should review all images and pathology results related to the case. The radiologist should determine which imaging modality and localization technique will be used, keeping in mind the modality which best demonstrates the residual target lesion or biopsy clip and compatibility of the localization system with the chosen imaging modality (4). Image-guided percutaneous localizations can be performed using mammography, digital breast tomosynthesis, US, MRI, or, rarely, ductography or CT guidance. The patient must provide informed consent prior to the procedure.
Most lesions can be successfully localized with placement of a single device. Bracketing, or using two or more localization devices, can also be performed to aid removal of nonpalpable lesions spanning 2.5–5.0 cm in extent and allow for a desired cosmetic outcome following surgery (5,6). Challenging cases, such as those involving bracketing, highlight the importance of completing pre-procedural review in collaboration with the surgeon to ensure the optimal localization procedure is performed (5,7).
Once the localization device is placed the radiologist may annotate post-procedure images or directly communicate with the surgeon. The radiologist may be asked to indicate the distance from skin entry site to the lesion and device or use an alternative labeling system preferred by the surgeon (5).
Following surgical excision, a specimen radiograph is often performed to confirm excision of the targeted lesion (7). Oftentimes, the radiologist will discuss the specimen radiograph findings with the pathologist and/or surgeon.
Wire Localization
Device Information
Wire localization devices have been used for presurgical localization of nonpalpable breast lesions for several decades. In 1976, Dr Howard Frank, a chest surgeon, developed the first breast WL device, the Frank hookwire (8). This hookwire consisted of a 25-gauge spinal needle preloaded with a wire that could be placed percutaneously and targeted to a specific lesion with the use of imaging guidance to confirm appropriate placement. Once in place, the Frank hookwire could not be repositioned. Several modifications have been made over subsequent years. The Kopans spring hookwire and the Homer J-wire allow for repositioning of the placement needle prior to deployment of the localization wire (8). The Kopans spring hookwire also allows for removal of the external placement needle, leaving only the flexible wire in place. Further modifications led to advancements that increased wire palpability and decreased the likelihood of wire transection at the time of surgery (8). Currently, multiple WL devices are available, including wires held in place by hooks, barbs, and pigtails (9).
Wire localization systems are available in various needle lengths (3–15 cm) with sterile, single-use introducers (16–20 gauge) (7). Wire localization devices are placed at or within 5 mm of the targeted breast lesion by deploying an internal flexible wire through an external stiff needle (4). After the introducer needle tip is positioned at the target the wire is advanced until the midpoint of the stiffener, or thickest part of the wire, is at the lesion, with the tip of the wire located 1–2 cm deep to the lesion (4,10). Bracketing with WL devices has been successful in the removal of larger breast lesions (10). Unlike some NWL devices, which should not be placed within 2 cm from one another to ensure differential detection, multiple wires can be placed within close proximity without risk of interference (Figure 1) (10). When appropriate needle placement is confirmed by imaging, the external stiff needle of many of these devices can be removed, leaving the flexible wire in place to mark the targeted lesion. The distal aspect of the wire protrudes externally through the skin, serving as a guide for the surgeon.
Figure 1.
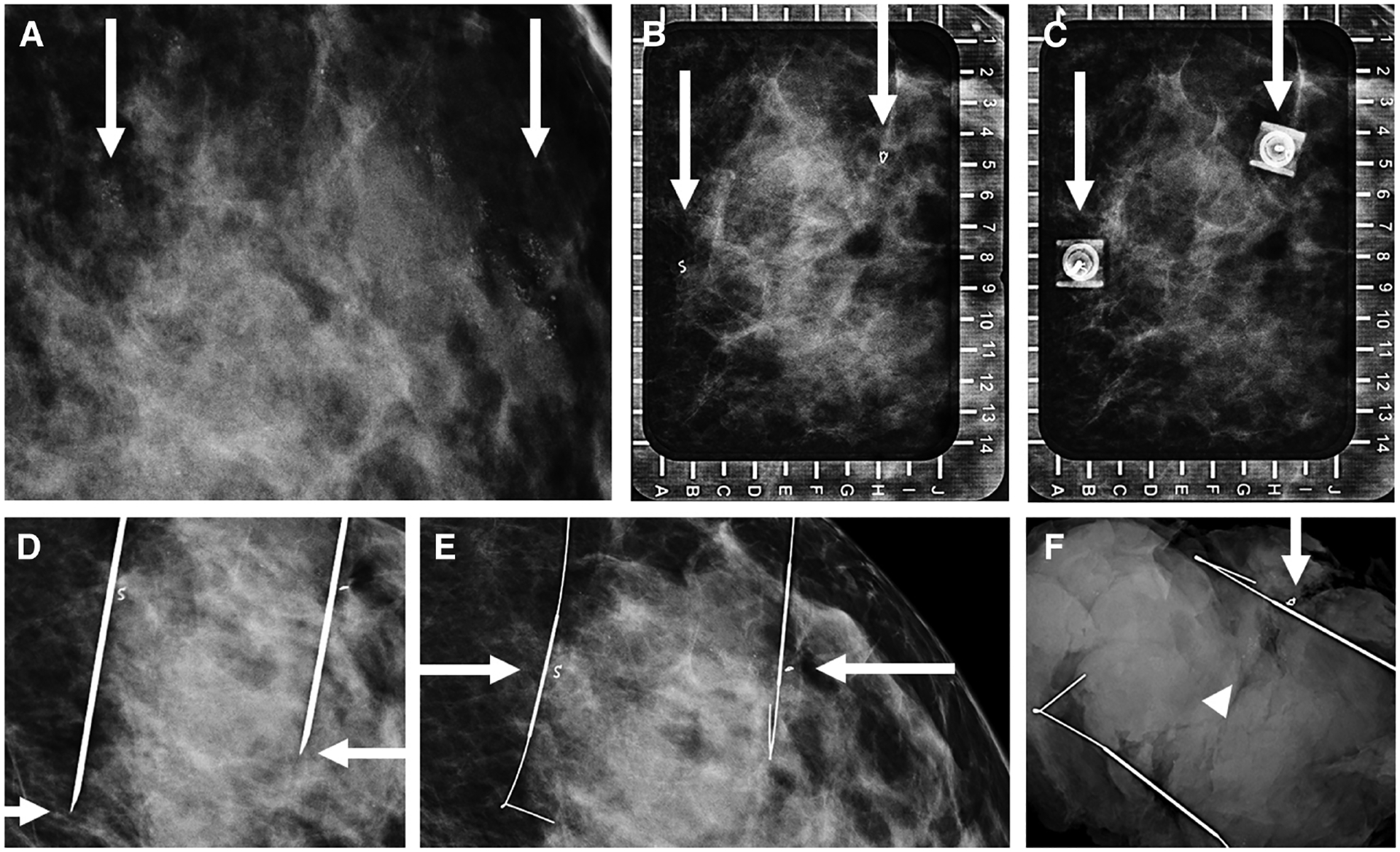
Images of a 42-year-old woman with biopsy-proven atypical ductal hyperplasia (ADH) who presented for bracketed wire localization. Magnification view of the left breast (A) shows round and amorphous calcifications spanning 2.8 cm (arrows). B: Alphanumeric grid for wire localization using a lateral approach for localizing the calcifications, marked by biopsy clips (arrows). Repeat image (C) shows both hubs of the wire needle overlying the two biopsy clips (arrows). Orthogonal craniocaudal view (D) confirms the tip of each needle to be distal (arrows) to the targeted biopsy clips. Repeat view (E) after the needles were removed confirms the thickest part of the wire to be located immediately adjacent to both biopsy clips (arrows). Specimen radiograph (F) shows removal of both wires, one biopsy clip (arrow) and residual calcifications (arrowhead) in the specimen. The findings were communicated to the surgeon who stated that the second clip was removed while suctioning during the excision. Final pathology yielded residual ADH without upgrade to malignancy.
Outcomes
Following percutaneous placement, wire migration has been reported in approximately 0%–1.8% of cases (11–13). The rate of positive margins in surgeries performed following WL range from 5.5% to 57% (3,11,14–18). Average surgical times for operative excision following WL range from 6 to 62 minutes (11,15,17). Positive margins and repeat operations, regardless of localization method, are more likely to occur in specimens containing ductal carcinoma in situ (DCIS) (19,20).
Benefits and Limitations
The benefits of WL include decades of proven reliability, effectiveness, and relatively low cost (3,7,12) Compared to most NWL techniques, WL is an economical choice, with an average device cost of $20 (3,7,12). Wire localization devices have a specific advantage over NWL devices as they can be placed under MRI guidance (7,21). Unlike some of the newer localization devices there is no limitation to the depth that can be reached with a WL device (22).
Institutions must also consider the drawbacks related to the WL workflow, which are primarily related to the necessity of performing WL on the same day of surgery. This scheduling linkage between WL and surgery can result in challenges coordinating radiology and surgery scheduling, exclusion from the first operative case of the day, delayed surgery start times, and unexpected radiology delays, leading to inefficiencies and unexpected costs (23,24). However, a recent feasibility study demonstrated that WL could be performed the day before surgery without significant wire migration, which may serve as a future option to overcome scheduling challenges (10).
Wire migration is another drawback to WL. Once placed, the external portion of the wire, although secured, carries a small risk of migration and dislodgement as the patient undergoes post-procedural imaging and transportation to the operative suite (24). While uncommon, in extreme cases wire migration has been associated with rare complications, such as pneumothorax, mediastinal perforation, and breast implant rupture (7,25–27). Wire localization is infrequently used for axillary lesions, as wire migration due to arm movement and muscular contraction may lead to potential damage to the adjacent axillary artery, vein, and the brachial plexus (28).
The external wire component may also impact surgical planning and approach for a small subset of surgeons (11,15). Those who use the wire entry site to make a surgical incision may find the percutaneous WL entry site less than ideal for the surgical approach (11,15). Transection and kinking of wires during surgery has also been reported (21,29,30).
As patients must be fasting at the time of WL for same-day surgery, lightheadedness and vasovagal episodes during localization have been reported (31). It has been suggested that patients undergoing WL experience higher levels of anxiety and decreased satisfaction when compared to patients undergoing NWL procedures (32,33).
Nonwire Localization
Multiple NWL devices are currently available for preoperative localization. These devices include radioactive seeds, radar reflectors, magnetic seeds, and radiofrequency identification tag localizers (RFIDs). Nonwire localization devices have been used to accurately localize unifocal breast lesions and axillary lymph nodes and to bracket larger radiologic lesions (7). Image-guided percutaneous NWL can be performed using mammography, digital breast tomosynthesis, US, or, rarely, ductography or CT guidance. The only NWL device that has been approved for MRI-guided localization is the radioactive seed. However, relevant safety precautions should be reviewed prior to localization (34,35).
Nonwire localization devices provide an opportunity to decouple radiology and surgery schedules and reduce operative and radiology delays and overall cost (17). However, most nonwire systems require an initial infrastructure acquisition cost, which is generally in the tens of thousands of dollars, with ongoing costs associated with nonreusable devices (10). It has been suggested that these costs are offset by operative cost savings due to decoupling of radiology and surgery schedules. However, further financial validation is needed (21).
Nonwire localization devices also improve the ease with which surgeons can confirm exact lesion location (17). Further, they allow radiologists to place the device without concern for the surgical approach. As the surgical approach is not directed by the path of a wire, there is potential for decreasing specimen volumes and improving cosmesis (36). However, unlike WL, NWL devices cannot be repositioned once deployed, occasionally necessitating an additional NWL or WL device to be placed (21).
Nonwire localization techniques allow for increased patient scheduling flexibility as they can be placed prior to or on the same day as surgery (36). Radar reflectors, magnetic seeds, and RFIDs have been approved for long-term implantation with intent to remove and can be placed in the breast or axilla prior to neoadjuvant chemotherapy. Long-term use also creates an area of future research in which an NWL device could be placed in lieu of a biopsy clip for highly suspicious lesions, thereby eliminating the need for a separate localization procedure (1). Finally, patient comfort is improved as an external wire component is not present and fasting is not required (if the NWL device is placed prior to the day of surgery), also decreasing the likelihood of a vasovagal reaction (36).
I-125 Radioactive Seed
Device Information
In 2001, I-125 radioactive seeds, in nontherapeutic doses, showed initial efficacy for localization of nonpalpable breast lesions and have since been used to localize axillary lymph nodes (11,29). While the life of a radioactive seed is brief, precautions are paramount for radioactive seed localization (RSL) programs to ensure patient safety and regulatory compliance. The I-125 coated radioactive material is encased within a 4.5 × 0.8-mm titanium seed and dosages range from 3.7–11.1 MBq (0.1–0.3 mCi), respectively (14,22). I-125 has a half-life of 59.4 days with a 27-keV gamma photon radiation peak (22). Commercially available preloaded radioactive seeds are commonly effective for 90 days with dose at the time of implantation calculated based on interval decay. Following receipt and package survey, activity is logged following Nuclear Regulatory Commission (NRC) guidelines and institutional protocols (22,37).
The seed is preloaded into a sterile 18-gauge needle with bone wax at the needle tip to avoid nontarget release and seed migration following deployment (11,37,38). A 10-cm standard needle size allows for localization in a variety of breast sizes and lesion locations. Other needle lengths are also available (5–15 cm) (22). Prior to initiation of the procedure, the radioactive seed needle should be checked with a Geiger-Muller (GM) survey meter to ensure that a seed is present (37). Once at its desired location, the seed is deployed by removing a rubber stopper and simultaneously pushing the internal stylet forward while pulling the needle hub back to uncover the seed at the target. Anecdotally, it has been found to be helpful to rotate the needle hub to ensure the seed and sterile bone wax are fully displaced from the needle tip and to avoid migration when the needle is removed. Following localization and prior to disposal of any procedure-related items, the localization site in the breast and the needle previously containing the seed are checked by GM to confirm implantation (37).
The differences in photopeak of the seed and the technitium-99 used for sentinel lymph node biopsy allow for concurrent use with an intraoperative gamma probe for differential detection. If technitium-99 sentinel lymph node injection is planned it should be performed following seed placement to avoid confusion, as the GM meters used in the radiology suite generally cannot differentiate between radioactive seed I-125 and technitium-99 activities (37).
Bracketing with seeds has been successful; however, an approximate distance of 2 cm between seeds is recommended to ensure differential detection. If close lesions require localization an alternative method, in conjunction with RSL, may be considered (Figures 2 and 3). There have been mixed results when comparing positive margin rates between bracketed WL and RSL techniques (39,40). Seeds should not be placed within hematomas or the hydrophilic component of a sonographically visible biopsy clip to avoid migration or removal by suction during surgery (14,22).
Figure 2.
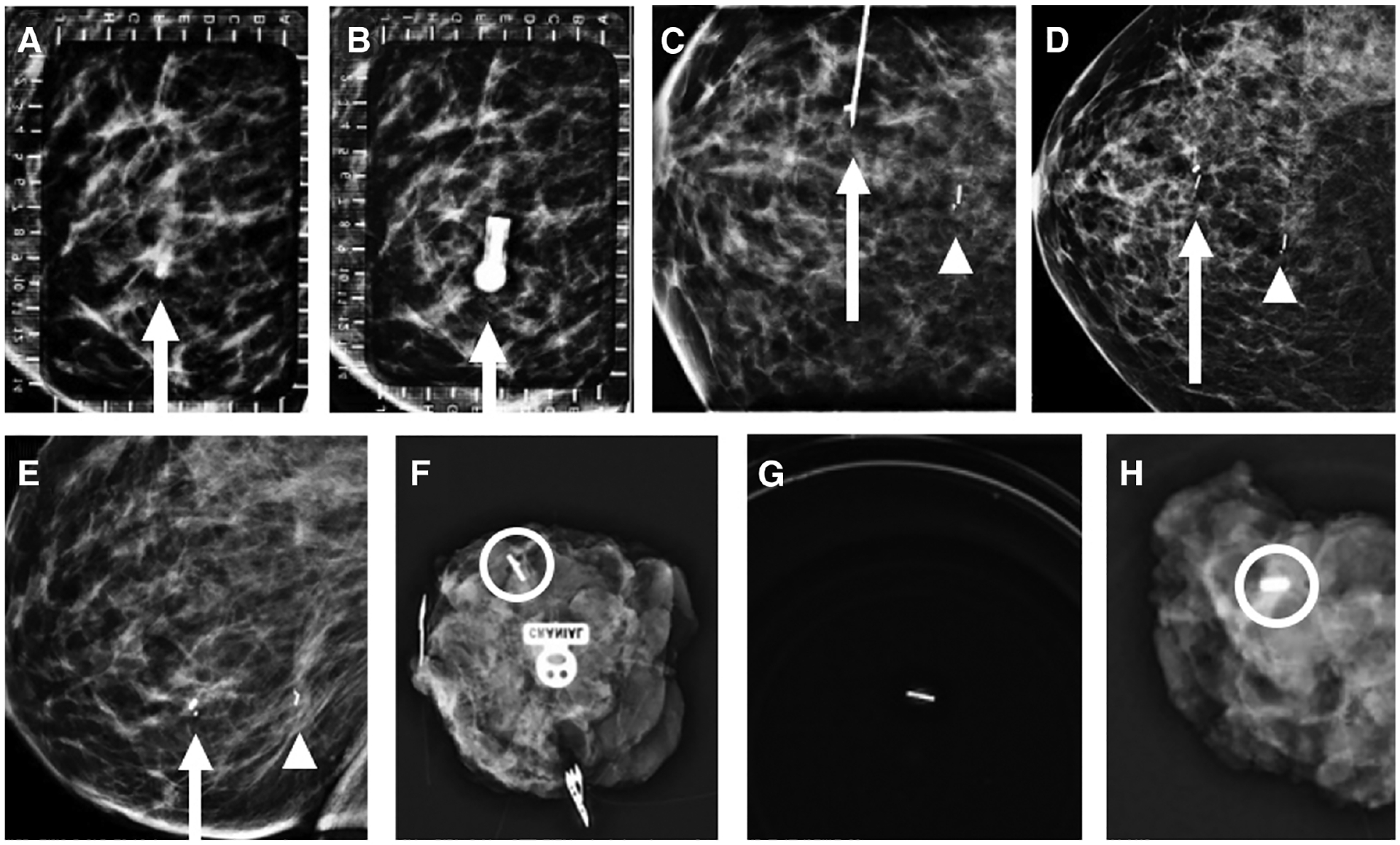
Images of a 48-year-old woman with biopsy-proven right breast invasive ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS), metastatic to the right axilla, status post-neoadjuvant chemotherapy. A: Alphanumeric grid for radioactive seed localization of a cork biopsy marker (arrow) localizing DCIS diagnosed via MRI-guided biopsy. Localization from a lateral approach (B) shows the hub of the radioactive seed localization needle overlying the biopsy marker (arrow). Orthogonal craniocaudal (CC) view (C) confirms that the tip of the radioactive seed localization needle (arrow) is adjacent to the cork biopsy marker prior to seed deployment. A second seed from US-guided radioactive seed localization of biopsy-proven IDC associated with a butterfly marker (arrowhead) is also imaged. Post–radioactive seed localization procedure CC (D) and mediolateral (E) views of the right breast confirms that seeds are adjacent to the biopsy markers (arrows and arrowheads) localizing sites of biopsy-proven malignancy. The first right breast specimen radiograph (F) shows one seed and an overlapping butterfly clip (circle) at the site of IDC. Additional right breast specimen radiographs show a seed in a separate specimen cup (G) to confirm retrieval due to dislodgement at excision and an additional specimen radiograph (H) with the cork biopsy clip (circle) at the site of DCIS.
Figure 3.
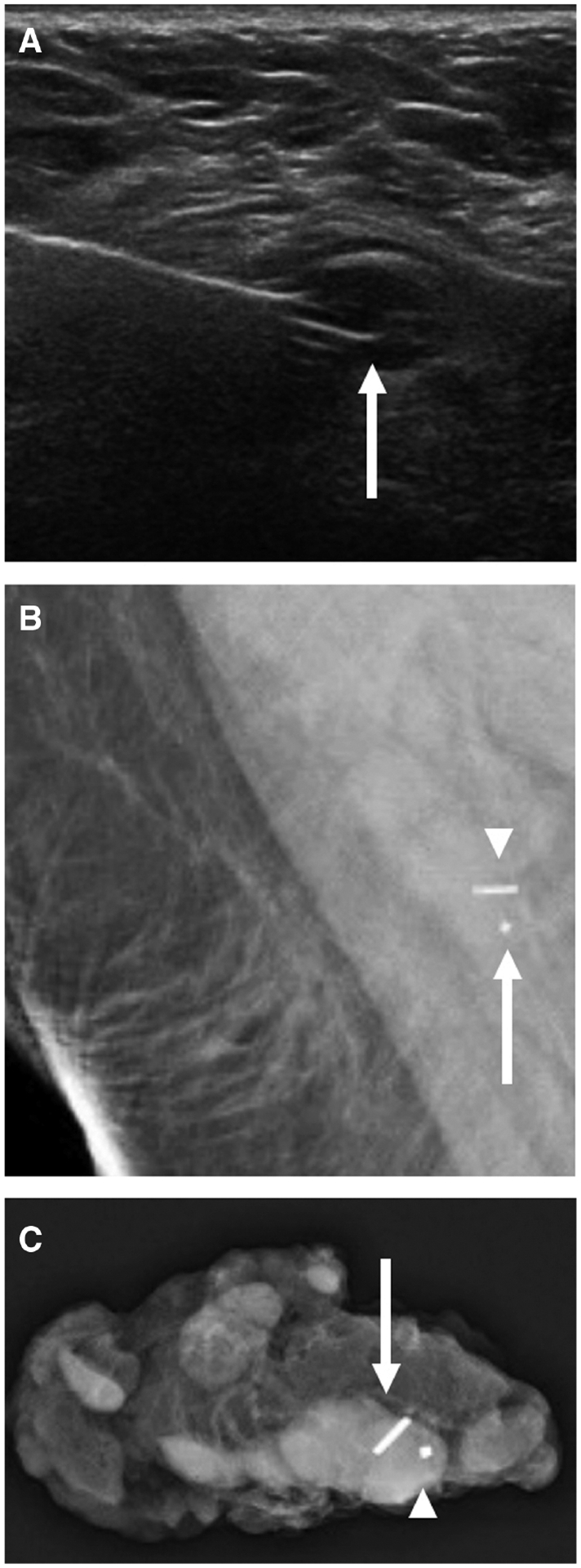
Images of a 48-year-old woman with biopsy-proven right breast invasive ductal carcinoma with ductal carcinoma in situ, metastatic to the right axilla, status post-neoadjuvant chemotherapy (same patient in Figure 2). A: US-guided radioactive seed localization of a biopsy-proven metastatic level 1 right axillary lymph node, with the tip of the needle deploying the seed in the lymph node (arrow). Post-procedural right breast mammogram coned down to the axilla (B) shows the seed (arrowhead) immediately adjacent to the biopsy clip (arrow). Specimen radiograph of the right axilla (C) shows the metastatic lymph node containing the marker (arrowhead) and seed (arrow).
Explantation of seeds is usually performed within five days after implantation. However, time to explantation is based on institutional protocol and the individual NRC license agreement (up to 14 days) (11,38). At the time the patient returns for surgery, an intraoperative gamma probe confirms seed activity and localizes the seed and lesion (37). Following excision, the surgical specimen and surgical cavity are checked for seed activity. At pathology, the specimen is checked for activity to avoid transection during sectioning. The radioactive seed is returned to radiology and safely secured during decay and then disposed of, returned to the manufacturer, or transferred to a licensed radioactive waste disposal facility (37,41).
Outcomes
Device migration is rare following percutaneous placement, ranging from 0.1% to 2%, and is most often related to placement technique or placement in a post-biopsy hematoma (14,42,43). Surgical outcomes with RSL are equivalent or superior to standard WL when comparing rates of positive excision margins and re-excision, ranging from 5% to 32% (11,14,15,19,20,24,29,42–46). Operative times range from 5.4 minutes to greater than 100 minutes with variable operative procedures (11,24,38,43,47).
Benefits and Limitations
Benefits of RSL are related to patient satisfaction, workflow efficiency, and cost savings. Several studies have shown that patients prefer RSL over WL, particularly when the localization is performed prior to the day of surgery (11,44,48). Radioactive seed localization also has cost savings related to greater workflow efficiencies, including an increase in scheduled radiology biopsy slot utilization and decreased time scheduling and rescheduling radiological and surgical procedures (17,29,47). The negligible cost differences between RSL and WL are primarily related to radiation survey instruments (ie, intraoperative gamma probes), which many institutions may already have for technetium-99 sentinel lymph node localization (47).
The primary barrier to implementation of RSL programs is regulation. In 2016, the U.S. NRC issued revised guidance to address the use of radioactive seeds for the purpose of breast surgical localization (41). Modifications included authorized user (AU) training and experience requirements, the need to work under the supervision of an AU, appropriate use of radiation surveys and instrumentation, and understanding of the criteria of a medical event (37). Medical event criteria include (1) use of the wrong radionuclide; (2) implantation of a radioactive seed into the wrong patient; (3) implantation of the wrong number of seeds; and (4) failure to perform explantation surgery (except in the setting of the patient failing to return for surgery or if seed removal may jeopardize patient health) (37). Of note, implantation into the wrong site does not include migration or off-target placement (37). In such an event, placement of additional seeds would not necessarily meet the criterion of “wrong number of seeds” but would necessitate communication between the radiologist and surgeon, as any implanted radioactive seed must be surgically removed from the breast (29,37).
An emergency protocol should be established in the unlikely event of seed breakage, leakage, or loss and widely instituted across involved disciplines (22,37). Should seed breakage and leakage occur while it is implanted in a patient, a radiation dose assessment and saturation of the patient’s thyroid with stable iodine must be considered to avoid irreversible damage (37). Seed loss can happen at the time of percutaneous placement, between time of implantation and surgery (particularly if placed in a superficial location), during surgery, or when handling the surgical specimen. Radiologists, surgeons, and pathology personnel should complete radiation training to ensure rapid identification of a lost or missing seed.
Radar Reflector
Device Information
The radiofrequency reflector localization device, SAVI SCOUT (Cianna Medical, Aliso Viejo, CA), was initially approved by the U.S. Food and Drug Administration (FDA) in 2014 for localization of nonpalpable breast lesions and has since been approved for axillary lymph node localization (21,49). The device is composed of an infrared-activated electromagnetic wave reflector that transmits an electrical signal between two nitonol antennae attached by way of a resistor (50). The electromagnetic waves produced can be audibly detected by a handpiece and console system.
The device is 12 mm in length and is housed in a sterile 16-gauge needle, available in three lengths (5 cm, 7.5 cm, and 10 cm) (50). Ideally, the 16-gauge introducer needle tip should be advanced approximately 6 mm distal to the center of the target, as the SAVI SCOUT is deployed by a withdrawal release, unsheathing the reflector to the center of the target (7). The antennae, located at each end of the device, help anchor the device at the site of deployment and prevent migration (4). The SAVI SCOUT has been approved for placement as deep as 6 cm from the skin surface. This depth is best measured sonographically with the patient in the supine position (21,51,52).
Bracketing with SAVI SCOUT has been successful, allowing for 2 cm between the localized lesions to ensure each reflector is identified separately at the time of surgery (7) (Figure 4). Following the procedure, SAVI SCOUT functionality is audibly detected and confirmed by way of a handpiece and console system.
Figure 4.
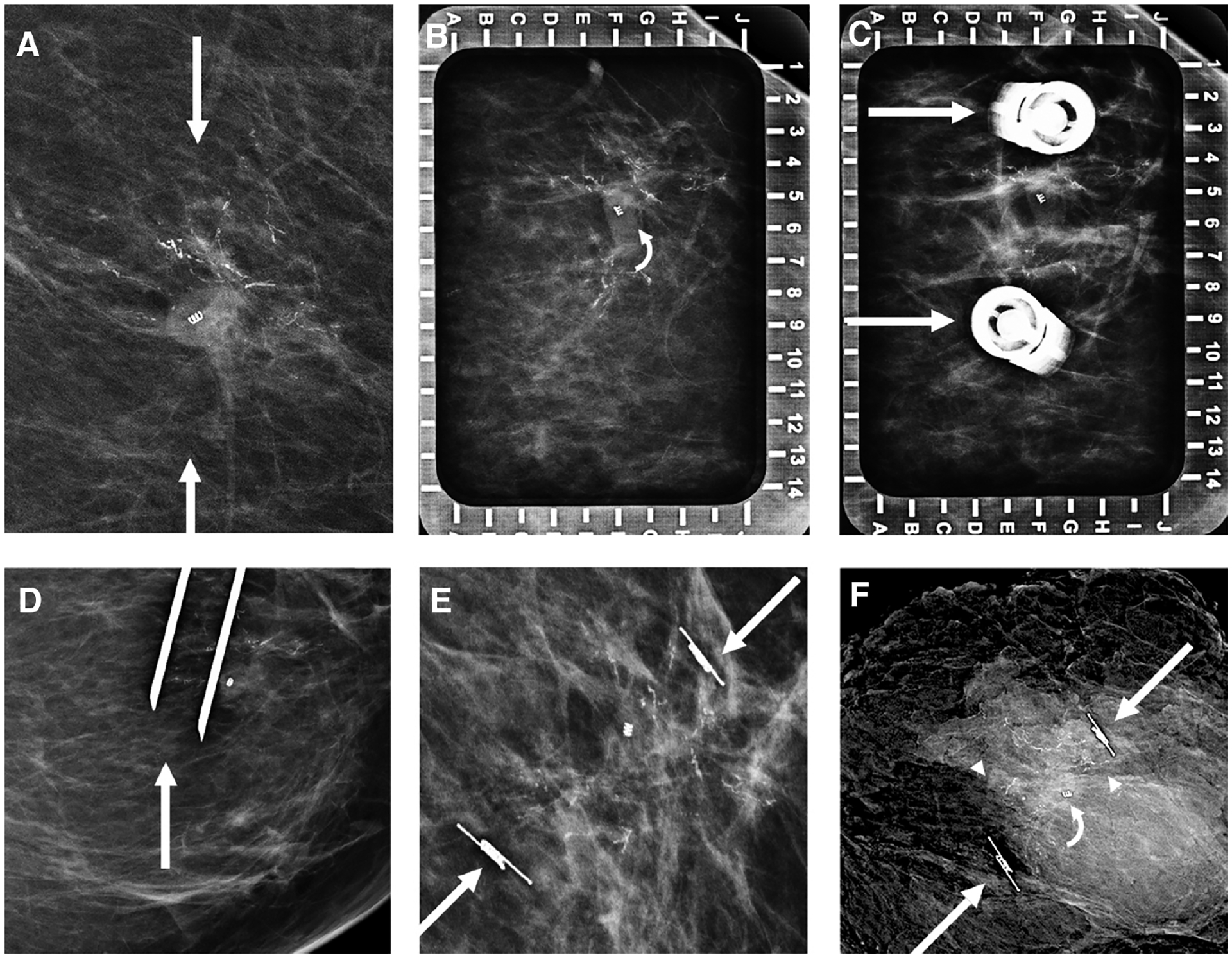
Images of a 43-year-old woman with biopsy-proven left breast ductal carcinoma in situ (DCIS). A: Bracketing SAVI SCOUT localization targeting residual calcifications (arrows) surrounding an open coil biopsy marker was requested. Alphanumeric grid (B) for bracketing from a superior approach shows residual calcifications surrounding the open coil biopsy clip (curved arrow). Repeat imaging (C) shows the SAVI SCOUT needle hubs (arrows) at the medial and lateral edges of residual calcifications. Orthogonal mediolateral projection (D) confirms the needle tips (arrow) to be approximately 6 mm distal to the inferior residual calcifications just prior to SAVI SCOUT deployment. Craniocaudal mammogram (E) confirms accurate placement of the SAVI SCOUT devices (arrows) bracketing the residual calcifications and biopsy marker. Specimen radiograph (F) shows residual calcifications (arrowheads), the biopsy marker (curved arrow), and SAVI SCOUT devices (arrows). At final pathology, the patient was upgraded to invasive ductal carcinoma, not otherwise specified, with residual DCIS.
Outcomes
Following percutaneous placement, device migration, defined as end location greater than 1.0 cm from the target, has been reported in approximately 4.5%–7.0% of cases, most commonly as a result of post-procedural hematoma (4,49,50,53). Positive margin rates following SAVI SCOUT localization range from 0% to 16.8% (18,49,50,52–54). A feasibility study demonstrated that, after performing five cases, surgeons reported the average operative time using SAVI SCOUT to be equal to that of WL (53).
Benefits and Limitations
SAVI SCOUT has been well-received among surgeons, radiologists, and patients. The audible electromagnetic feedback allows the surgeon to reorient around a point source, decreasing removal of healthy tissue (53). One survey showed that surgeons preferred SAVI SCOUT over WL for ease of surgical approach and confidence in removing the desired target (54). The same survey reported that 71% of patients were very satisfied with SAVI SCOUT and 97% would recommend SAVI SCOUT to others (54). SAVI SCOUT can be imaged in a 1.5 or 3 T magnetic field with little to no resultant susceptibility artifact, a considered advantage over other NWL devices, which demonstrate susceptibility artifact at time of MRI, potentially limiting evaluation of response to neoadjuvant therapy (36).
Cost is likely the most important consideration prior to adopting SAVI SCOUT. The initial cost associated with purchasing reusable consoles and probes for radiology and surgery suites is tens of thousands of dollars. Ongoing costs are associated with nonreusable reflectors, averaging $450 per device (36).
A reported disadvantage of SAVI SCOUT is difficulty with detection if placed greater than 6 cm deep from the skin surface, within a hematoma, or posterior to a dense breast mass such as a calcified fibroadenoma. These pitfalls can be overcome by repositioning the breast to decrease the volume of overlying tissue and placing the reflector adjacent to, rather than within or behind, a hematoma or dense breast mass. Although rare, it has been reported that signal detection may be absent when the reflector comes into direct contact with the intraoperative electrocautery device or is exposed to older halogen operating room lights (49). SAVI SCOUT contains nickel and should be avoided in patients with a documented nickel allergy (50).
Magnetic Seed
Device Information
Magseed (Endomagnetics Inc., Cambridge, UK) is an inducible, non-radioactive, magnetic device initially approved for use by the FDA in 2016 for breast localization and since approved for axillary lymph node localization (55). Magseed is composed of paramagnetic iron oxide and stainless steel, cylindrical in shape, and measures 5 × 1 mm (56). The seed is preloaded into a sterile 18-gauge stainless steel deployment needle, available in 7 cm and 12 cm lengths, and is stabilized inside the deployment needle by a bone wax plug (56–58). Ideally, the 18-gauge introducer needle tip should be advanced to the center of, or immediately adjacent to the target, as a steel obturator is advanced within the introducer needle, pushing the seed forward during deployment (Figure 5) (56). Bracketing with Magseed has been successfully performed, allowing for 2 cm between the localized lesions to ensure that each seed is identified at the time of surgery (57).
Figure 5.
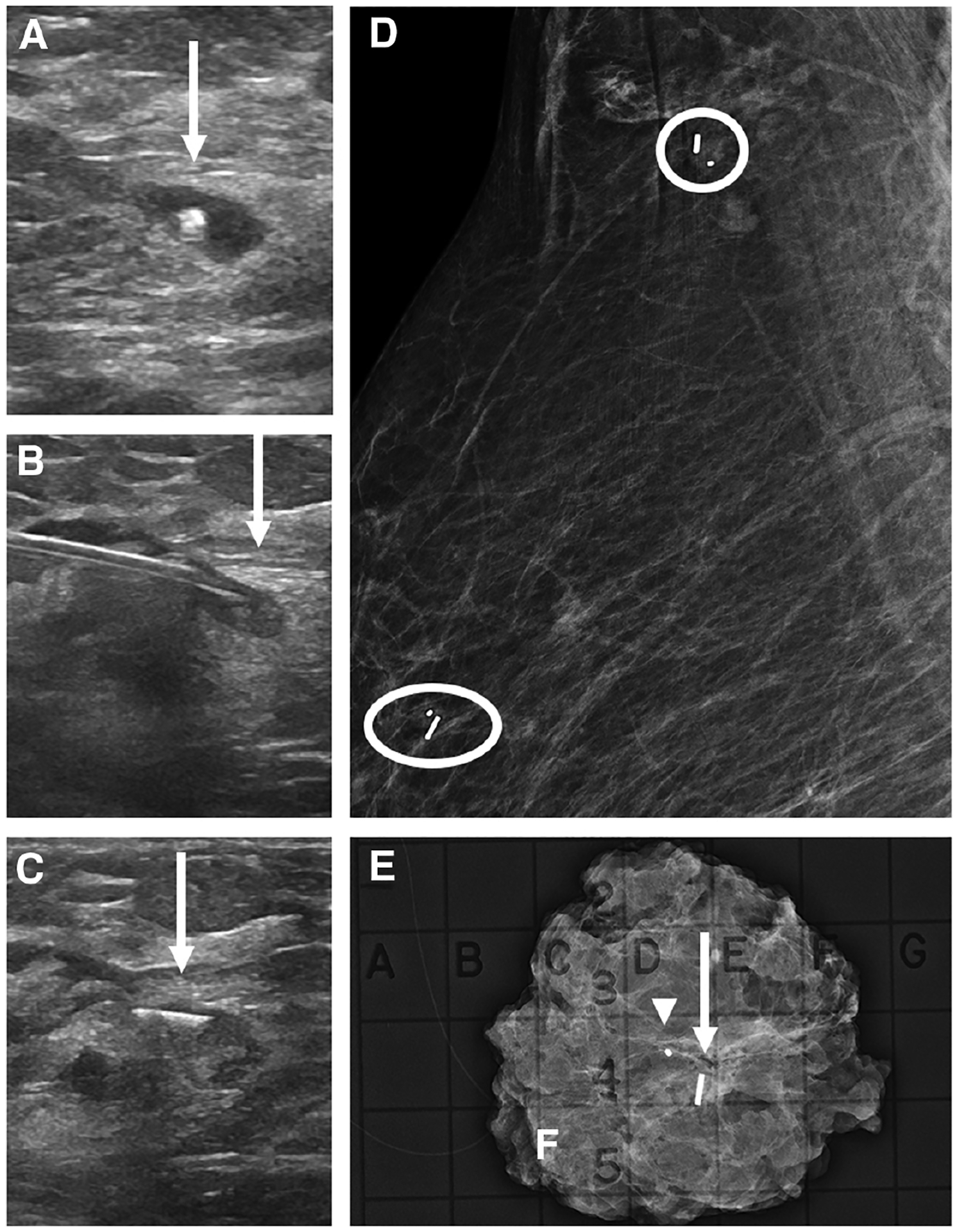
Images of a 66-year-old woman with biopsy-proven right breast invasive ductal carcinoma with lobular features, metastatic to the right axilla, presenting for breast and axillary Magseed localization following neoadjuvant chemotherapy. Right breast US (A) shows a residual mass in the right breast with a central biopsy clip (arrow). Right breast US (B) shows the Magseed needle with the tip in the residual mass (arrow) prior to deployment. Following deployment, US (C) shows the Magseed within the superior edge of the residual mass (arrow). Post-procedural right breast mediolateral oblique mammogram (D) shows the Magseed adjacent to the biopsy clip in the upper breast (oval). A second Magseed was also placed under US guidance at the biopsy-proven level 1 axillary lymph node containing a biopsy marker (circle). Specimen radiograph of the right breast (E) shows the marker (arrowhead), Magseed (arrow), and residual surrounding focal asymmetry. The axillary specimen was not submitted for review.
Intraoperatively, Magseed is transiently magnetized using the Sentimag detector probe (Endomagnetics Inc., Cambridge, UK), which generates an alternating magnetic field current (55). The Sentimag probe detects the seed’s magnetization and displays audio and numerical feedback, with the sound frequency and numerical value increasing as the probe approaches the seed (55).
The manufacturer reports that the Magseed sensing depth is up to 4 cm from the skin surface. However, sensing depths greater than 4 cm utilizing intraoperative detector compression and palpation have been achieved (57,58). Singh et al reported Magseed retrieval of all 19 lesions greater than 3 cm deep from the skin surface with surgeons able to localize these lesions “very or fairly easily” in nearly all cases (59). Additionally, Harvey et al found that Magseed was detectable in all breast sizes, with superficial seed placement and smaller breast size correlating to decreased localization time and higher numerical detection counts intraoperatively (56).
Outcomes
Following percutaneous placement, device migration, defined as end location greater than 1.0 cm from the target, has been reported in approximately 0%–4.5% of cases (33,56,57,59–62). The rate of positive margins requiring re-excision following Magseed localization ranges from 9% to 22%, with no statistically significant difference in re-excision rates when directly comparing Magseed and WL (55). One prospective study demonstrated operative localization time ranging from 4 to 47 minutes, with similar operative time for patients with more than one Magseed placed (59).
Benefits and Limitations
Magseed has been well-received among surgeons, radiologists, and patients. The audible and numerical feedback allow the surgeon to continuously reorient around a point source, decreasing removal of healthy tissue (61). One survey demonstrated that 93% of surgeons rated ease of transcutaneous localization as “very or fairly easy” and 87% rated ease of localization following incision as “very or fairly easy” (59). The same survey reported that 61% of radiologists rated Magseed as “very easy” to place (59). One study reported that patients reported lower anxiety scores with Magseed localization when compared with WL (32).
A potential limitation of Magseed utilization is the initial cost of Magseed units and Sentimag probes when compared to WL and radioactive seeds (60). Ongoing costs associated with individual devices average $400. Additional purchasing costs can accrue, as non-ferromagnetic intraoperative equipment should be used to eliminate interference with the magnetic localization signal and Sentimag detector probe (60).
Currently, Magseed cannot be inserted using MRI guidance but is considered MRI conditional (55). However, Magseed produces the largest reported susceptibility artifact (approximately 4 cm) and, if placed prior to neoadjuvant treatment, the artifact may limit the radiologist’s ability to accurately assess residual disease (9,55).
Radiofrequency Identification Tag
Device Information
Radiofrequency identification tag localization (LOCalizer RFID; Hologic, Inc., Marlborough, MA) was introduced in 2017 and uses radio wave signals to identify breast lesions for surgical excision (63). It is currently FDA-approved to remain in the breast long-term (>30 days). The system includes an 11 × 2-mm RFID tag with a unique identification number (63). The tag is composed of a ferrite rod wrapped in copper and a microprocessor enclosed in a glass casing within an antimigratory polypropylene sheath (7,63). The tag is preloaded within a sterile 12-gauge needle applicator, available in various lengths (5 cm, 7 cm, and 10 cm). The 12-gauge introducer needle tip should be advanced to the center of, or immediately adjacent to the target, as a steel obturator is advanced within the introducer needle, pushing the seed forward during deployment (Figure 6).
Figure 6.
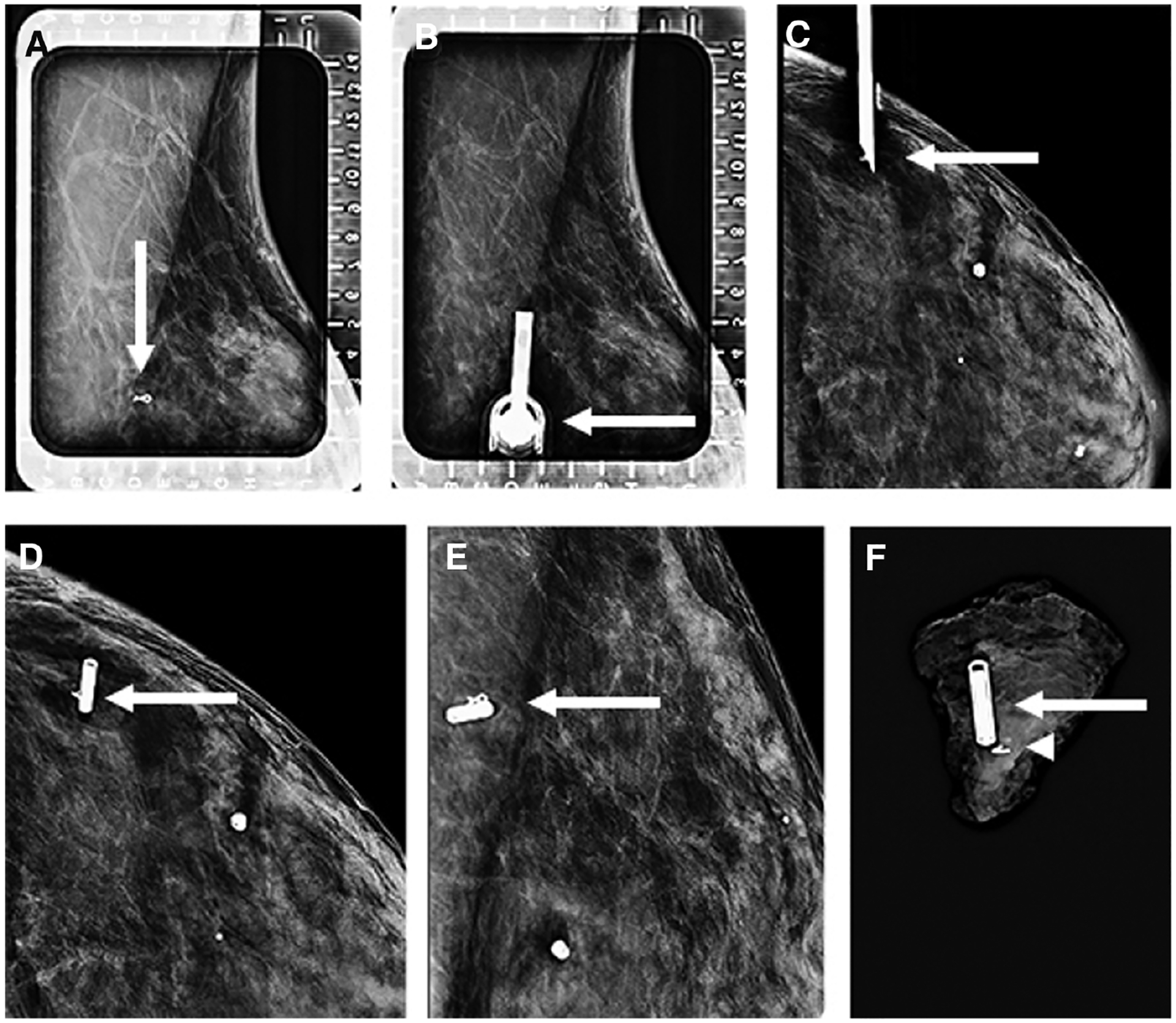
Images of a 44-year-old women status post-neoadjuvant therapy for left breast invasive ductal carcinoma, not otherwise specified, who presented for radiofrequency identification (RFID) tag localization of a ribbon clip marking the site of the treated cancer. Left breast alphanumeric grid for localization from a lateral approach (A) demonstrates the ribbon clip (arrow). Repeat image (B) shows the needle housing the RFID tag inserted with the hub (arrow) directly overlying the ribbon clip. Orthogonal craniocaudal (CC) view (C) demonstrates the needle tip (arrow) at the depth of the ribbon clip before deployment. The RFID tag was subsequently deployed, with the CC (D) and mediolateral (E) views demonstrating the RFID tag (arrow) to be adjacent to the ribbon clip. Specimen radiograph (F) confirms that the ribbon clip (arrowhead) and RFID tag (arrow) are within the surgical specimen.
After percutaneous placement and at the time of surgical excision, a handheld reader uses an audible sound that increases as the device approaches the tag, as well as a visual indicator on its screen showing the tag’s distance from the detector, up to 60 mm. The reader also displays the tag’s unique identification number, which can be helpful when more than one tag is implanted in the same breast (7,63). A pencil-sized, single-use, sterile surgical probe guides the surgeon towards the tag intraoperatively and can detect the tag within a distance of 40 mm (63).
Outcomes
Preliminary data demonstrate high success rates for localization and retrieval with negligible migration rates prior to surgery (0%–0.6%) (64). To our knowledge, there has only been one reported case of tag migration reported in which a tag placed via ultrasound guidance immediately migrated back along the introducer track (64).
A small number of studies have reported early success using tag localization for marking metastatic axillary lymph nodes treated with neoadjuvant chemotherapy (65,66). Laws et al reported a high retrieval rate (39/40) of successfully localized axillary lymph nodes, which is comparable to rates using RSL in the axilla (65).
RFID has similar positive margin rates (0%–27%) and operative times compared to other methods of localization (31,34,62,64,67–69). In a study by McGugin et al there was no significant difference between tag and WL in terms of overall specimen volume, operative times, and re-excision rates, although there was some variability based on lesion type (69). For instance, they found that the re-excision rate for DCIS was higher for lesions bracketed with tags than with wires (42.9% vs 7.1%). They also found that if two tags were placed closer than 1.8 cm their unique IDs would not appear on the tag reader due to interference, making it difficult for the surgeon to accurately locate the tags, and concluded that wires may be preferable to tags for short-distance bracketed procedures (69).
Benefits and Limitations
Although comparatively new, RFID has demonstrated multidisciplinary ease of use and relatively high patient satisfaction. Data have shown that surgeons and radiologists often prefer tag localization to other methods of localization (31,62,66,68). One study reported surgeon preference for RFID over Magseed as the RFID probe was noted to be lighter and easier to handle with more precise navigation and accurate estimation of distance from the probe to the tag (62). The small caliber (8-mm tip) of the surgical probe can be used in small incisions without obscuring visualization of the target (7). Also, there is no need to remove metal instruments from the surgical field and the tag does not interfere with electrocautery (34,62). In another study, four surgeons gave a 100% satisfaction rating for the tag’s ability to localize the target in surgery and all four recommended the product (70). Patients have also given tag localization high satisfaction scores (31,62). DiNome et al reported that most patients agreed or strongly agreed (94%) that their tag localization went smoothly and agreed or strongly agreed (78%) that the procedure was easier than expected (31).
Reported challenges with the RFID system include difficulty with placement, given the applicator’s large gauge, especially within dense breasts (64,70). Some have recommended placing a small skin incision prior to placement (66). Anecdotal reports suggest that creating a tract with a similarly gauged introducer device prior to placing the needle is helpful in tag placement. Furthermore, as the tag is enclosed in glass, it may break during surgery (66). Although MRI is possible with the tag in situ, the tag creates a significant artifact on MRI (artifact reported to span 2–5 cm), which would limit MRI evaluation of malignancies following neoadjuvant therapy (34,64). Last, the cost of the tag and disposable probe is approximately $550 per patient, making this localization method more expensive than WL.
Conclusion
Preoperative, image-guided localization aids surgeons in identifying nonpalpable breast and axillary lesions and achieving negative margins at the time of excision. As there are no significant differences in surgical outcomes when comparing WL and NWL devices, decisions regarding the selection and adoption of image-guided localization methods should be based on multidisciplinary discussions between surgeons and radiologists that consider several factors. Factors to consider include cost, workflow efficiency, physician preference, and ease of use. Further, as breast cancer patients are motivated advocates for value-based, high-quality care, patient satisfaction regarding different localization methods should be considered (Table 2). By understanding these factors, institutions ranging in size from small community practices to large tertiary care centers can make informed decisions on preferred localization techniques.
Key Messages.
Although there are no significant differences in surgical outcomes when comparing wire and nonwire localization devices, each localization method has unique benefits and drawbacks.
When selecting an optimal localization method for nonpalpable breast lesions, individual institutions should seek multidisciplinary input to select a device that promotes value-based, patient-centered care.
Factors to consider prior to the selection and adoption of an image-guided localization method include cost, workflow efficiency, physician preference and ease of use, and patient satisfaction.
Funding
This work was supported in part by the Vanderbilt-Ingram Cancer Center Support Grant (CA68485), the Vanderbilt-Ingram Cancer Center SPORE in Breast Cancer(P50CA098131), and the Vanderbilt-Ingram Cancer Center Ambassadors Discovery Grant in Cancer Research through the Kaye and Frank Delfino Caring Hearts Fund.
Footnotes
Conflict of Interest Statement
None declared.
References
- 1.Cheang E, Ha R, Thornton CM, Mango VL. Innovations in image-guided preoperative breast lesion localization. Br J Radiol 2018;91(1085):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyna C, DeSnyder SM. Intraoperative margin assessment in breast cancer management. Surg Oncol Clin N Am 2018;27(1):155–165. [DOI] [PubMed] [Google Scholar]
- 3.Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol 2013;2013(793819):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spalluto LB, DeBenedectis CM, Morrow MS, Lourenco AP. Advances in breast localization techniques: an opportunity to improve quality of care and patient satisfaction. Semin Roentgenol 2018;53(4):270–279. [DOI] [PubMed] [Google Scholar]
- 5.Butler R, Berg WA. Preoperative lesion localization, bracketing. In: Gelsinger AG, ed. Diagnostic Imaging: Breast. 3rd ed. Philadelphia, PA: Elsevier; 2019. [Google Scholar]
- 6.Parvez E, Cornacchi SD, Hodgson N, et al. A cosmesis outcome substudy in a prospective, randomized trial comparing radioguided seed localization with standard wire localization for nonpalpable, invasive, and in situ breast carcinomas. Am J Surg 2014;208(5):711–718. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor MM, Patel MM, Scoggins ME. The wire and beyond: recent advances in breast imaging preoperative needle localization. Radiographics 2019;39(7):1886–1906. [DOI] [PubMed] [Google Scholar]
- 8.Hall FM, Kopans DB, Sadowsky NL, Homer MJ. Development of wire localization for occult breast lesions: Boston remembrances. Radiology 2013;268(3):622–627. [DOI] [PubMed] [Google Scholar]
- 9.Hayes MK. Update on preoperative breast localization. Radiol Clin North Am 2017;55(3):591–603. [DOI] [PubMed] [Google Scholar]
- 10.Sanders LM, Morgan D, Polini N, Mehta A. Preoperative wire localization of the breast on the day before surgery. J Breast Imaging 2020;2(3):240–249. [DOI] [PubMed] [Google Scholar]
- 11.Gray RJ, Salud C, Nguyen K, et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol 2001;8(9):711–715. [DOI] [PubMed] [Google Scholar]
- 12.Jeffries DO, Dossett LA, Jorns JM. Localization for breast surgery: the next generation. Arch Pathol Lab Med 2017;141(10):1324–1329. [DOI] [PubMed] [Google Scholar]
- 13.Owen AW, Kumar EN. Migration of localizing wires used in guided biopsy of the breast. Clin Radiol 1991;43(4):251. [DOI] [PubMed] [Google Scholar]
- 14.Gray RJ, Pockaj BA, Karstaedt PJ, Roarke MC. Radioactive seed localization of nonpalpable breast lesions is better than wire localization. Am J Surg 2004;188(4):377–380. [DOI] [PubMed] [Google Scholar]
- 15.Lovrics PJ, Goldsmith CH, Hodgson N, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol 2011;18(12):3407–3414. [DOI] [PubMed] [Google Scholar]
- 16.Dauer LT, Thornton C, Miodownik D, et al. Radioactive seed localization with 125I for nonpalpable lesions prior to breast lumpectomy and/or excisional biopsy: methodology, safety, and experience of initial year. Health Phys 2013;105(4):356–365. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Seely J, Cordeiro E, et al. Radioactive seed localization versus wire-guided localization for nonpalpable breast cancer: a cost and operating room efficiency analysis. Ann Surg Oncol 2017;24(12):3567–3573. [DOI] [PubMed] [Google Scholar]
- 18.Patel SN, Mango VL, Jadeja P, et al. Reflector-guided breast tumor localization versus wire localization for lumpectomies: a comparison of surgical outcomes. Clin Imaging 2018;47:14–17. [DOI] [PubMed] [Google Scholar]
- 19.Law W, Cao X, Wright FC, Slodkowska E, Look Hong N, Curpen B. Adequacy of invasive and in situ breast carcinoma margins in radioactive seed and wire-guided localization lumpectomies. Breast J 2021;27(2):134–140. [DOI] [PubMed] [Google Scholar]
- 20.Wang GL, Tsikouras P, Zuo HQ, et al. Radioactive seed localization and wire guided localization in breast cancer: a systematic review and meta-analysis. J BUON 2019;24(1):48–60. [PubMed] [Google Scholar]
- 21.Mayo RC III, Kalambo MJ, Parikh JR. Preoperative localization of breast lesions: current techniques. Clin Imaging 2019;56:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Goudreau SH, Joseph JP, Seiler SJ. Preoperative radioactive seed localization for nonpalpable breast lesions: technique, pitfalls, and solutions. Radiographics 2015;35(5):1319–1334. [DOI] [PubMed] [Google Scholar]
- 23.Loving VA, Edwards DB, Roche KT, et al. Monte Carlo simulation to analyze the cost-benefit of radioactive seed localization versus wire localization for breast-conserving surgery in fee-for-service health care systems compared with accountable care organizations. AJR Am J Roentgenol 2014;202(6):1383–1388. [DOI] [PubMed] [Google Scholar]
- 24.Murphy JO, Moo TA, King TA, et al. Radioactive seed localization compared to wire localization in breast-conserving surgery: initial 6-month experience. Ann Surg Oncol 2013;20(13):4121–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopans DB. Breast Imaging. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 26.Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol 2014;202(4):W390–W399. [DOI] [PubMed] [Google Scholar]
- 27.Azoury F, Sayad P, Rizk A. Thoracoscopic management of a pericardial migration of a breast biopsy localization wire. Ann Thorac Surg 2009;87(6):1937–1939. [DOI] [PubMed] [Google Scholar]
- 28.Woods RW, Camp MS, Durr NJ, Harvey SC. A review of options for localization of axillary lymph nodes in the treatment of invasive breast cancer. Acad Radiol 2019;26(6):805–819. [DOI] [PubMed] [Google Scholar]
- 29.Sharek D, Zuley ML, Zhang JY, Soran A, Ahrendt GM, Ganott MA. Radioactive seed localization versus wire localization for lumpectomies: a comparison of outcomes. AJR Am J Roentgenol 2015;204(4):872–877. [DOI] [PubMed] [Google Scholar]
- 30.Homer MJ. Transection of the localization hooked wire during breast biopsy. AJR Am J Roentgenol 1983;141(5):929–930. [DOI] [PubMed] [Google Scholar]
- 31.DiNome ML, Kusske AM, Attai DJ, Fischer CP, Hoyt AC. Microchipping the breast: an effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat 2019;175(1):165–170. [DOI] [PubMed] [Google Scholar]
- 32.Small S, McAdam A, Mathers H. P156. Analysis of patient anxiety related to Magseed and guide-wire localisation techniques. Eur J Surg Oncol 2019;45(5):925. [Google Scholar]
- 33.Thekkinkattil D, Kaushik M, Hoosein MM, et al. A prospective, single-arm, multicentre clinical evaluation of a new localisation technique using non-radioactive Magseeds for surgery of clinically occult breast lesions. Clin Radiol 2019;74(12):974.e7–974.e11. [DOI] [PubMed] [Google Scholar]
- 34.Lee MK, Sanaiha Y, Kusske AM, et al. A comparison of two non-radioactive alternatives to wire for the localization of non-palpable breast cancers. Breast Cancer Res Treat 2020;182(2):299–303. [DOI] [PubMed] [Google Scholar]
- 35.Lee C, Bhatt A, Felmlee JP, et al. How to safely perform magnetic resonance imaging-guided radioactive seed localizations in the breast. J Clin Imaging Sci 2020;10(19):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalambo M, Parikh JR. Implementing the SAVI SCOUT system in community radiology practice. J Am Coll Radiol 2017;14(9):1234–1238. [DOI] [PubMed] [Google Scholar]
- 37.Sheetz M, Steiner C. Compliance with the U.S. nuclear regulatory commission revised licensing guidance for radioactive seed localization. Health Phys 2018;115(3):402–408. [DOI] [PubMed] [Google Scholar]
- 38.Stelle L, Schoenheit T, Brubaker A, et al. Radioactive seed localization versus wire localization for nonpalpable breast lesions: a two-year initial experience at a large community hospital. Ann Surg Oncol 2018;25(1):131–136. [DOI] [PubMed] [Google Scholar]
- 39.Da Silva M, Porembka J, Mokdad AA, et al. Bracketed radioactive seed localization vs bracketed wire-localization in breast surgery. Breast J 2018;24(2):161–166. [DOI] [PubMed] [Google Scholar]
- 40.Srour MK, Kim S, Amersi F, Giuliano AE, Chung A. Comparison of multiple wire, radioactive seed, and Savi Scout® radar localizations for management of surgical breast disease. Ann Surg Oncol 2021;28:2212–2218. [DOI] [PubMed] [Google Scholar]
- 41.Iodine-125 and palladium-103 low dose rate brachytherapy seeds used for localization of non-palpable lesions. NRC web. Available at: https://www.nrc.gov/materials/miau/med-use-toolkit/seed-localization.html. Accessed February 23, 2021. [Google Scholar]
- 42.McGhan LJ, McKeever SC, Pockaj BA, et al. Radioactive seed localization for nonpalpable breast lesions: review of 1000 consecutive procedures at a single institution. Ann Surg Oncol 2011;18(11):3096–3101. [DOI] [PubMed] [Google Scholar]
- 43.van Riet YE, Jansen FH, van Beek M, van de Velde CJ, Rutten HJ, Nieuwenhuijzen GA. Localization of non-palpable breast cancer using a radiolabelled titanium seed. Br J Surg 2010;97(8):1240–1245. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JH, Mason MC, Gray RJ, et al. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. Breast J 2008;14(2):153–157. [DOI] [PubMed] [Google Scholar]
- 45.Xu XJ, Li JJ, Ji WB. An updated meta-analysis of radioactive seed localization versus wire-guided localization in the treatment of nonpalpable breast lesions. Breast J 2018;24(4):673–675. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed M, Douek M. Radioactive seed localisation (RSL) in the treatment of non-palpable breast cancers: systematic review and meta-analysis. Breast 2013;22(4):383–388. [DOI] [PubMed] [Google Scholar]
- 47.Diego EJ, Soran A, McGuire KP, et al. Localizing high-risk lesions for excisional breast biopsy: a comparison between radioactive seed localization and wire localization. Ann Surg Oncol 2014;21(10):3268–3272. [DOI] [PubMed] [Google Scholar]
- 48.Bloomquist EV, Ajkay N, Patil S, Collett AE, Frazier TG, Barrio AV. A randomized prospective comparison of patient-assessed satisfaction and clinical outcomes with radioactive seed localization versus wire localization. Breast J 2016;22(2):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasem I, Mokbel K. Savi Scout® radar localisation of non-palpable breast lesions: systematic review and pooled analysis of 842 cases. Anticancer Res 2020;40(7):3633–3643. [DOI] [PubMed] [Google Scholar]
- 50.Mango VL, Wynn RT, Feldman S, et al. Beyond wires and seeds: reflector-guided breast lesion localization and excision. Radiology 2017;284(2):365–371. [DOI] [PubMed] [Google Scholar]
- 51.Falcon S, Weinfurtner RJ, Mooney B, Niell BL. SAVI SCOUT® localization of breast lesions as a practical alternative to wires: outcomes and suggestions for trouble-shooting. Clin Imaging 2018;52:280–286. [DOI] [PubMed] [Google Scholar]
- 52.Jadeja PH, Mango V, Patel S, et al. Utilization of multiple SAVI SCOUT surgical guidance system reflectors in the same breast: a single-institution feasibility study. Breast J 2018;24(4):531–534. [DOI] [PubMed] [Google Scholar]
- 53.Mango V, Ha R, Gomberawalla A, Wynn R, Feldman S. Evaluation of the SAVI SCOUT surgical guidance system for localization and excision of nonpalpable breast lesions: a feasibility study. AJR Am J Roentgenol 2016;207(4):W69–W72. [DOI] [PubMed] [Google Scholar]
- 54.Cox CE, Russell S, Prowler V, et al. A prospective, single arm, multi-site, clinical evaluation of a nonradioactive surgical guidance technology for the location of nonpalpable breast lesions during excision. Ann Surg Oncol 2016;23(10):3168–3174. [DOI] [PubMed] [Google Scholar]
- 55.Gera R, Tayeh S, Al-Reefy S, Mokbel K. Evolving role of Magseed in wireless localization of breast lesions: systematic review and pooled analysis of 1559 procedures. Anticancer Res 2020;40(4):1809–1815. [DOI] [PubMed] [Google Scholar]
- 56.Harvey JR, Lim Y, Murphy J, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat 2018;169(3):531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price ER, Khoury AL, Esserman LJ, Joe BN, Alvarado MD. Initial clinical experience with an inducible magnetic seed system for preoperative breast lesion localization. AJR Am J Roentgenol 2018;210(4):913–917. [DOI] [PubMed] [Google Scholar]
- 58.Magseed Marker—Magnetic Lesion Localization | Mammotome, Neoprobe, Sentimag. Mammotome Available at: https://www.mammotome.com/magseed/. Accessed February 14, 2021. [Google Scholar]
- 59.Singh P, Scoggins ME, Sahin AA, et al. Effectiveness and safety of Magseed localization for excision of breast lesions: a prospective, phase IV trial. Ann Surg Open 2020;1(2):e008. doi: 10.1097/AS9.0000000000000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamb LR, Bahl M, Specht MC, D’Alessandro HA, Lehman CD. Evaluation of a nonradioactive magnetic marker wireless localization program. AJR Am J Roentgenol 2018;211(4):940–945. [DOI] [PubMed] [Google Scholar]
- 61.Zacharioudakis K, Down S, Bholah Z, et al. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol 2019;45(11):2016–2021. [DOI] [PubMed] [Google Scholar]
- 62.Tayeh S, Gera R, Perry N, Michell M, Malhotra A, Mokbel K. The use of magnetic seeds and radiofrequency identifier tags in breast surgery for non-palpable lesions. Anticancer Res 2020;40(1):315–321. [DOI] [PubMed] [Google Scholar]
- 63.LOCalizer™ wire-free guidance system | hologic. Available at: https://www.hologic.com/hologic-products/breast-skeletal/localizer-wire-free-guidance-system. Accessed February 9, 2021. [Google Scholar]
- 64.Lowes S, Bell A, Milligan R, Amonkar S, Leaver A. Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Clin Radiol 2020;75(12):942–949. [DOI] [PubMed] [Google Scholar]
- 65.Laws A, Dillon K, Kelly BN, et al. Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol 2020;27(12):4819–4827. [DOI] [PubMed] [Google Scholar]
- 66.Malter W, Eichler C, Hanstein B, Mallmann P, Holtschmidt J. First reported use of radiofrequency identification (RFID) technique for targeted excision of suspicious axillary lymph nodes in early stage breast cancer—evaluation of feasibility and review of current recommendations. In Vivo 2020;34(3):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol 2015;204(6):W720–W723. [DOI] [PubMed] [Google Scholar]
- 68.Wazir U, Tayeh S, Perry N, Michell M, Malhotra A, Mokbel K. Wireless breast localization using radio-frequency identification tags: the first reported European experience in breast cancer. In Vivo 2020;34(1):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGugin C, Spivey T, Coopey S, et al. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res Treat 2019;177(3):735–739. [DOI] [PubMed] [Google Scholar]
- 70.Malter W, Holtschmidt J, Thangarajah F, et al. First reported use of the Faxitron LOCalizer™ radiofrequency identification (RFID) system in Europe—a feasibility trial, surgical guide and review for non-palpable breast lesions. In Vivo 2019;33(5):1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]