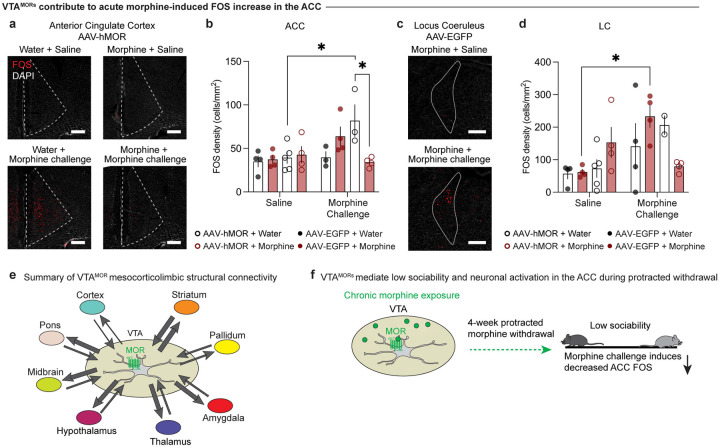
Figure 6: VTAMORs contribute to acute morphine-induced FOS increase in the ACC.
FOS density following a saline or morphine challenge injection (20 mg/kg, s.c.) in the (a, b) anterior cingulate cortex (ACC) (three-way ANOVA + Bonferroni’s multiple comparison, treatment F1,23=0.5316, p=0.4733; virus F1,23=0.8245, p=0.3733; injection F1,23=7.059, p=0.0141; interaction (injection x virus x drinking F1,23=8.982, p=0.0064, scale, 200 μm) and (c, d) locus coeruleus (LC) (three-way ANOVA + Bonferroni’s multiple comparison, treatment F1,23=0.2204, p=0.6431; virus F1,23=0.02855, p=0.8673; injection F1,23=8.597, p=0.0075; interaction (injection x virus x drinking F1,23=7.507, p=0.0117, scale, 200 μm). In the ACC, a morphine challenge injection leads to increased (a, b) FOS in AAV-hMOR-injected opioid naïve mice, while morphine-treated mice show reduced FOS expression compared to their opioid naïve counterparts. In the LC, a morphine challenge injection leads to increased (c, d) FOS in AAV-EGFP-injected opioid naïve mice. (e) Schematic representation of VTAMOR neurons’ structural connectivity as explored across our VTAMOR input-output mapping experiments. Arrow thickness represents estimated connection density. (f) Schematic summary of morphine bound to a VTAMOR neuron and its subsequent effects on low sociability and morphine challenge-induced ACC FOS during protracted withdrawal. Data are represented as mean ± SEM, *p<0.05.