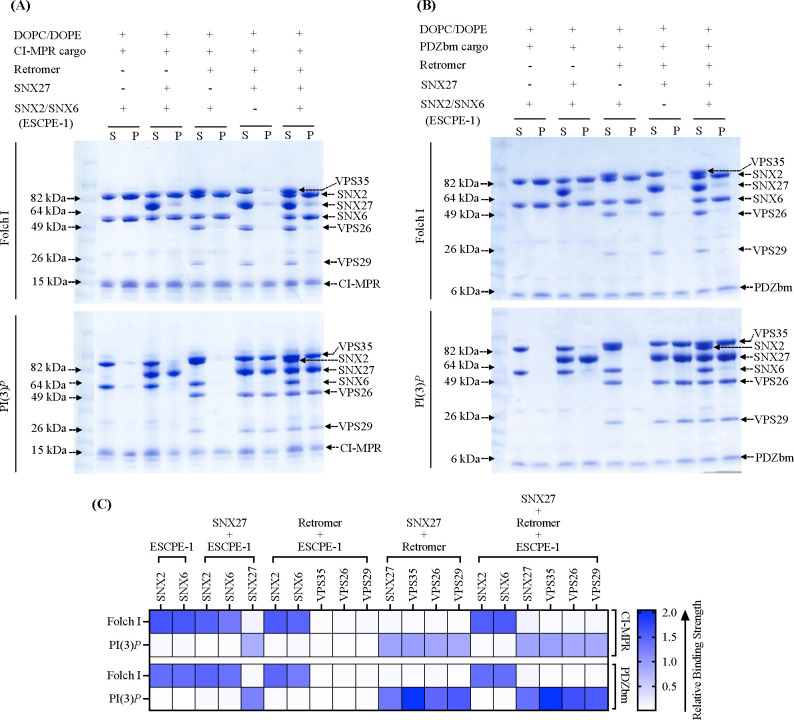
Figure 4. Biochemical reconstitution approaches reveal ESCPE-1 and SNX27/Retromer form different sub-complexes on membranes.
Purified recombinant human SNX2/SNX6 (ESCPE-1), SNX27, and Retromer were incubated with (A) CI-MPR cargo motif or (B) PDZbm cargo motif from 5HT4(a)R in the presence of Folch I- (upper Coomassie gel) or PI(3)P-enriched liposomes (lower Coomassie gel). Samples were subjected to ultracentrifugation followed by SDS-PAGE and Coomassie staining of unbound supernatant (S) and bound pellet (P) fractions. (C) Protein binding to phosphoinositide-enriched membranes visualized by SDS-PAGE was quantified by measuring relative protein band intensities (ImageJ). Reconstitution data reveal specificity of sorting nexin complexes for both phospholipid and cargo composition on liposome membranes. SNX2/SNX6 (ESCPE-1) robustly binds membranes enriched in Folch I and CI-MPR cargo motifs, while SNX27 binds membranes loaded with PI(3)P and PDZbm cargo motifs. SNX27 recruits Retromer, while mammalian SNX2/SNX6 (ESCPE-1) complex does not appear to recruit Retromer in the presence of either cargo or phospholipid.