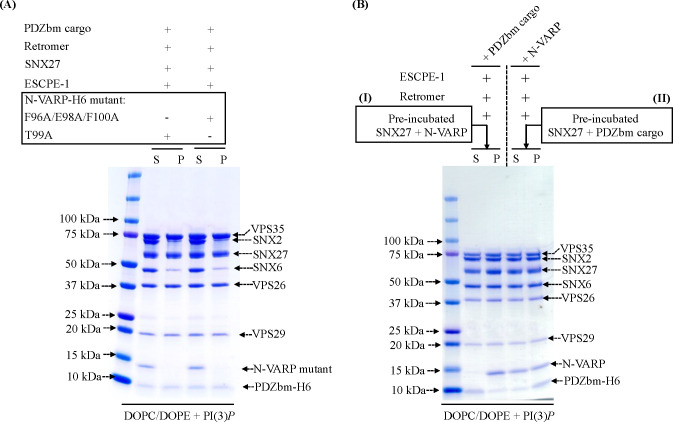
Figure 6. The VARP N-terminus is sufficient to recruit an endosomal supercomplex to membranes in vitro.
(A) Liposome pelleting experiments demonstrate VARP N-terminus mutants (residues 1–117) cannot recruit the endosomal supercomplex to membranes in vitro. Purified proteins of the N-VARP triple mutant (F96A/E98A/F100A) or single mutant (T99A) were incubated with SNX27, ESCPE-1, and Retromer in the presence of PDZbm cargo and PI(3)P-enriched liposomes. In both experiments, SNX27 and Retromer are recruited, but ESCPE-1 exhibits only partial binding to membranes. N-VARP mutants remain in the supernatant (S) fraction. (B) A competition experiment demonstrates how binding between N-VARP and SNX27 does not interfere with PDZbm cargo binding and membrane recruitment in the liposome pelleting assays. In experiment (I), full-length purified SNX27 protein was pre-incubated (see Methods) with purified N-VARP protein. All endosomal coat protein components are pelleted efficiently in the presence of wild-type N-VARP. SNX27, Retromer, and ESCPE-1 are found in the pellet (P) fraction in a ration similar to that observed for full-length VARP (Figure 5). In experiment (II), full-length purified SNX27 protein was pre-incubated with purified PDZbm-H6 peptide. All other endosomal proteins were pelleted efficiently in the presence of N-VARP. In experiment (II), there is a greater amount of cargo in the pellet fraction compared to experiment (I). These results together suggest N-VARP is sufficient to promote endosomal supercomplex formation on membranes and does not inhibit cargo binding or incorporation into the coat.