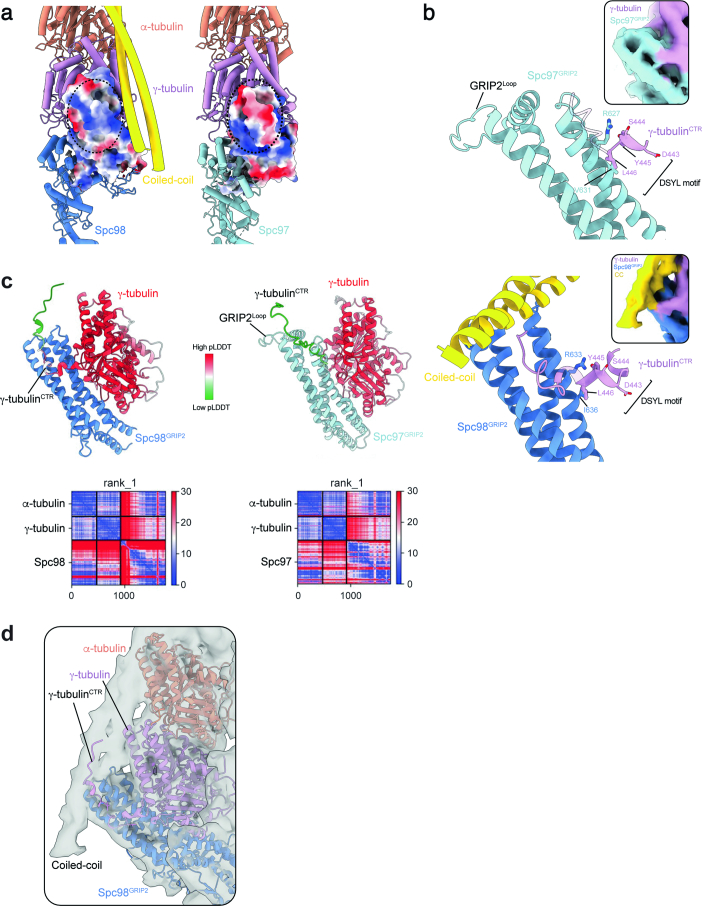
Extended Data Fig. 4. Spc98 presents a unique interface to bind the coiled-coil density.
a, Electrostatic surface potential of Spc98 and Spc97 in the γTuSC structure shows that the coiled-coil protein is lined against a positively charged patch on Spc98GRIP2. b, Alphafold2 predictions and cryo-EM density insets of the interface between the γ-tubulinCTR and Spc98GRIP2 (top panel) and Spc97GRIP2 (lower panel). The γ-tubulinCTR is predicted to bind extensively along the Spc98GRIP2 domain and extends beyond the conserved ‘DSYL’ motif towards the coiled-coil density. At the Spc97GRIP2 domain on the other hand, the γ-tubulinCTR tail is not predicted to interact beyond the ‘DSYL’ motif. c, γ-tubulin pLDDT scores mapped on the Alphafold2 predictions shown in panel b. γ-tubulin is coloured by the pLDDT score while Spc98 and Spc97 are coloured by subunit. PAE plots for the best model are also shown as insets. d, The unstructured γ-tubulinCTR extends toward the coiled-coil density in the γTuRC average.