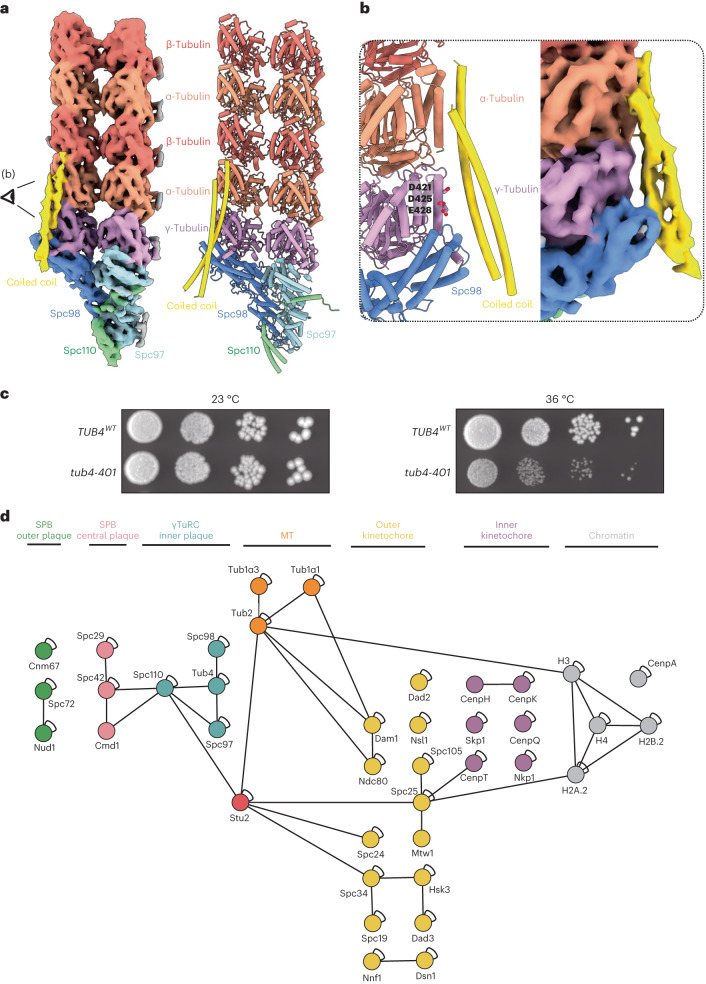
Fig. 3. A coiled-coil protein spans the Spc98:γ-tubulin:α-tubulin interface.
a, A subboxed cryo-ET map at 8 Å, and molecular model of a γTuSC bound to the first two rows of α/β-tubulin. b, A model and map of the coiled-coil interaction with Spc98, γ-tubulin and α/β-tubulin. The crystal structure of the coiled-coil domain of Stu2 (ref. 38) fits the coiled-coil density in the subtomogram average, as shown in b. c, The tub4-401 mutant (γ-tubulin mutant D421R, E425R and E428R), predicted to disrupt the coiled-coil interaction with γ-tubulin, grows normally at 23 °C but shows growth defects at 36 °C. TUB4WT, wild-type γ-tubulin. d, Intra- and intermolecular cross-links observed during CLMS experiments of the enriched SPB preparation. Proteins are shown as nodes, labeled with their respective protein names, and color-coded according to their protein group affiliation (as indicated above). Cross-links identified from the listed protein groups through CLMS analysis of the enriched SPB preparation are depicted. Self-links are represented by loops on the nodes, while heteromeric cross-links are denoted by lines connecting the nodes. γTuRC proteins are labeled in dark cyan. Two coiled-coil proteins, Stu2 and BRE1, and one globular protein (Ima1) were found to be cross-linked to γTuRC proteins, of which Stu2, known to be enriched at SPBs, is shown in red.