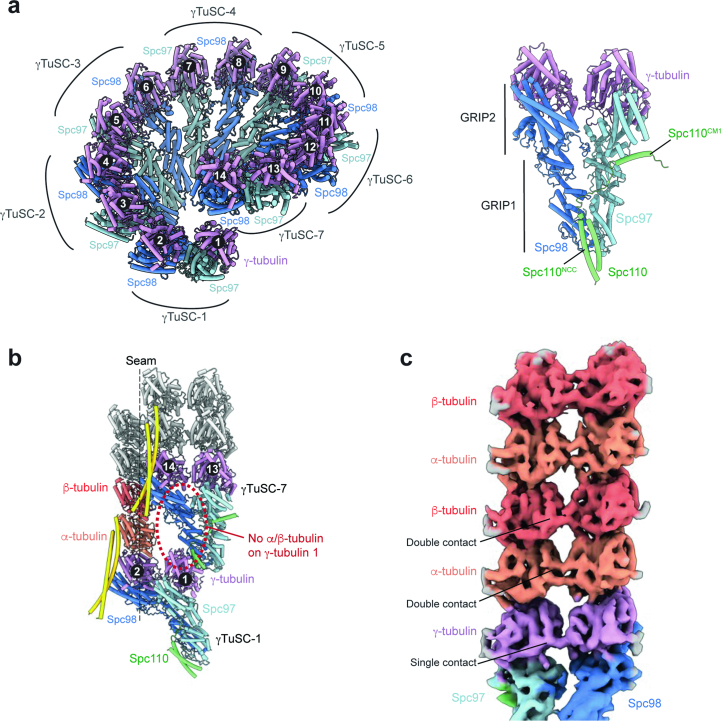
Extended Data Fig. 2. γTuRC geometry and interactions during MT nucleation.
a, Top-down view of γTuRC models with numbered γTuSCs and γ-tubulins (left). A molecular model of the γTuSC module with bound Spc110 protein and annotated GRIP1 and GRIP2 domains, is depicted on the right. b, Molecular model of the γTuRC seam shows that the first γ-tubulin bound to Spc97 is positioned precisely below the last Spc98 bound to the last (14th) γ-tubulin, with a large gap between them. This blocks α/β-tubulin binding to spoke 1 of γTuRC. c, Cryo-ET map of a sub-boxed γTuSC bound to the first two rows of α/β-tubulin, displaying the lateral α:α-tubulin (double contact), β:β-tubulin (double contact) and γ:γ-tubulin interfaces (single contact).