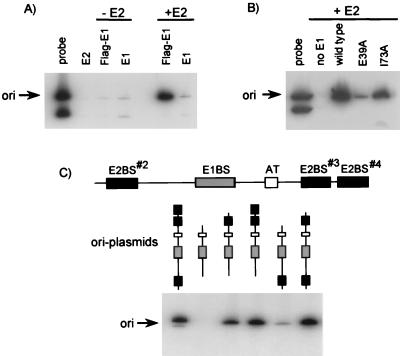
FIG. 2.
E2-dependent binding of E1 to the origin (ori). (A) Origin DNA-protein complexes were formed with E2 alone (E2) or with E1 that was either tagged at its N terminus with a FLAG epitope (Flag-E1) or untagged (E1). Complexes were formed either in the absence (−E2) or presence (+E2) of E2 and were immunoprecipitated with the M2 anti-FLAG monoclonal antibody (Eastman Kodak). Fifty percent of the coprecipitated DNA, along with 0.5% of the amount of probe used in the binding reaction, was visualized by electrophoresis and autoradiography. The arrow indicates the fragment of the probe that contains the origin. (B) Effect of the E39A and I73A substitutions in E2 on formation of the E1-E2-ori complex. Protein-DNA complexes were formed with E2 alone (no E1) or with a combination of E1 plus one of three E2 proteins: wild type, E39A mutant, or I73A mutant. The immunoprecipitation of complexes and detection of bound DNA were done as described for panel A. (C) Effect of deleting one, two, or all three E2-binding sites in the origin. A diagram of the HPV-11 origin is shown that highlights the three E2-binding sites (E2BS; solid boxes), the AT-rich region (AT; open box), and the palindromic E1-binding site (E1BS; shaded box). Complexes were formed with in vitro-translated E1 and E2 proteins and the indicated origin. Each probe was composed of two fragments (not shown), of which only the ori-containing fragment was coprecipitated. Similar amounts of each probe were used in these binding reactions (data not shown). The immunoprecipitation of complexes and detection of bound DNA were done as described for panel A.