Abstract
Introduction:
Racial/ethnic minorities have demonstrated a worse survival after allogeneic hematopoietic cell transplantation (HCT) compared to Whites. Whether the racial disparity in HCT outcomes persists in long-term survivors, and possibly may even be exacerbated in this population that frequently transitions back from the transplant center to their local healthcare providers, is unknown. In the current study we compared long-term outcomes among one-year allogeneic HCT survivors by race/ethnicity and socioeconomic status (SES).
Methods:
The Center for International Blood and Marrow Transplant (CIBMTR) database was used to identify 5,473 patients with acute myeloid leukemia, acute lymphocytic leukemia, chronic myeloid leukemia, or myelodysplastic syndromes who received first allogeneic HCT between 2007–2017 and were alive and in remission for at least 1 year after transplantation. Study was restricted to patients transplanted in the United States. SES was defined using patient neighborhood poverty level estimated from the recipient’s ZIP code of residence; a ZIP code with ≥20% of persons below the federal poverty level was considered a high poverty area. The primary outcome was to evaluate the association of race/ethnicity and neighborhood poverty level with overall survival (OS), relapse, and non-relapse mortality (NRM). Cox regression models were used to determine associations of ethnicity/race and SES with (OS), relapse, and (NRM). Standardized mortality ratios (SMR) were calculated to compare mortality rates of the study patients to their general population peers matched on race/ethnicity, age and sex.
Results:
Patients were reported to be Non-Hispanic White (n=4,385), Non-Hispanic Black (n=338), Hispanic (n=516), and Asian (n=234). Overall, 729 (13%) patients resided in areas with high poverty level. We found that a significantly larger proportion of non-Hispanic Black (37%) and Hispanic (26%) patients lived in areas with higher poverty levels compared to Non-Hispanic Whites (10%) and Asians (10%) (p<0.01). In multivariable analysis, we observed no significant association between OS, PFS, relapse, or NRM and race/ethnicity or poverty level when adjusted for patient-, disease-and transplant-related covariates.
Conclusions:
Our retrospective cohort registry study highlights that among adult allogeneic HCT recipients who survived at least 1-year in remission, there was no observed association between race/ethnicity, neighborhood poverty level, and long-term outcomes.
INTRODUCTION
It has been shown racial/ethnic minorities and recipients residing in areas with higher neighborhood poverty levels and poorer community health status have worse survival after allogeneic hematopoietic cell transplantation (HCT) compared to Whites and patients without socioeconomic adversity1–5. Factors such as race, socioeconomic status (SES), and healthcare insurance have been shown to influence decisions regarding stem cell transplantation.6 There is limited information on access variations among different patient populations to HCT. Recent literature has tried to breakdown some disparities which may alter access to patients in need of HCT.2,7 The identified barriers to access were age, gender, race, SES, and insurance status.8,9 Even in the age of a growing geriatric oncology population, younger patients are still more likely to receive HCT.10 With respect to sex, men are more likely to have HCT than females. 11 Studies have also shown uninsured status, Medicaid or Medicare insurance lowers likelihood of HCT.12 In addition to these, race has also been identified as a significant factor affecting outcomes.10
Survivorship care focuses on the patient beyond treatment period and continues providing long term care after active treatment has ceased.13 In HCT patients, unique complications and therapy related events can occur even years after HCT 14,15. Many HCT patients are encouraged to maintain follow up with a transplant center for the duration of their lifetime.16 This can pose a unique challenge for HCT survivors such as: monitoring for disease recurrence, presence of graft-versus-host disease, long term chemotherapy associated effects.17 We chose the one-year landmark since this is the typical timeframe when care is transitioned from transplant center to community providers, especially those without significant graft versus host disease(GHVD). Outside of disease and treatment specific monitoring, survivorship can include assessments on quality of life, general health maintenance, and social and psychological adjustments after treatment.18 Causes of death after transplantation can be attributed to secondary malignancies, recurrent disease, infections, chronic GVHD, respiratory diseases, cardiovascular diseases, all associated with transplantation.19 Given racial minorities are more often from lower SES, these late effects from HCT may be accentuated in this vulnerable population.20 Without resources to integrate back into society after HCT, it is likely many of these patients are lost to follow up and do not follow the prescribed survivorship plan which may be a driver for worse outcomes.21
A previous large Center for International Blood and Marrow Transplant Research (CIBMTR) study investigated the association of race/ethnicity and SES with unrelated allogeneic HCT outcomes and showed that African American patients had worse overall survival (OS) after HCT compared to Whites.5 African Americans and Hispanics were also shown to have higher cumulative incidence of non-relapse mortality (NRM).5 Recipients from the lowest SES quartile had worse overall survival and higher NRM. Of note, the effects of race and SES on survival were independent of each other and it is important to note that the inferior outcomes among African Americans could not be explained by transplant-related factors or SES.22 Survival was considered from the time of transplantation, and this study did not specifically focus on long-term HCT survivors who typically are no longer under the direct care of transplant centers and could be more prone to disparities in care and outcomes.
To address the gaps in literature, our study investigated racial and SES outcome disparities in long-term allogeneic HCT survivors. We selected a representative, multicenter cohort of survivors from the CIBMTR database who were in remission for at least 1 year after allogeneic transplantation. We sought to: 1) determine association of ethnicity/race and neighborhood poverty level on survival among adult allogeneic HCT survivors with hematologic malignancies, 2) investigate the cumulative incidence of NRM and relapse post-transplant by ethnicity/race and neighborhood poverty level in this patient population, and 3) compare standardized mortality ratios (SMR) between our cohort and that of their age- and gender-matched peers in the general population.
MATERIALS AND METHODS
Data Source
The CIBMTR is a voluntary working group composed of nearly 500 transplantation centers worldwide that contribute detailed HCT data to a statistical center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by onsite audits. Patients are followed longitudinally. Computerized checks for discrepancies, physician reviews of submitted data, and onsite audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants and are under the guidance of the institutional review board of the NMDP.
Study Population
The study population consisted of first allogeneic HCT recipients from 2007–2017, age >18 years at transplant who were alive and in remission ≥1 years from HCT with the diagnosis of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), or myelodysplastic syndromes (MDS). The study was restricted to patients treated in the United States (US). All graft sources, donor sources, and conditioning regimens were considered. The CIBMTR database was used to identify 5,473 patients who met the study selection criteria and included 4,385 non-Hispanic White, 338 non-Hispanic Black, 516 Hispanic, and 234 Asian patients. Other race/ethnicity groups including American Indian/Alaskan Natives, Hawaiian/Pacific Islanders and mixed race were excluded because of the small number of patients in those categories to conduct meaningful analyses.
Statistical Analysis
The primary objective was to evaluate the association of race/ethnicity (non-Hispanic White (referent) vs. non-Hispanic Black vs. Hispanic vs. Asian) and neighborhood poverty level with OS, relapse, and NRM. Neighborhood poverty exposure was used to describe SES in our study and was defined per the US Census definition as living in a high-poverty ZIP code, with high poverty level consisting of ≥20% of persons below 100% federal poverty level 1. OS was defined as time from HCT to death from any cause. NRM was defined as time from HCT to death without relapse, with relapse being a competing event. Relapse was defined as time to recurrence of disease with NRM as a competing event. Patients were censored at date of last follow-up for all outcomes defined above. We also described the causes of death for our cohort.
Baseline characteristics were compared between racial and SES groups. OS was summarized using Kaplan-Meier method. Probabilities of NRM and relapse were calculated using cumulative incidence function. Cox proportional hazards regression model was used to evaluate the effect of the main variables of interest (race/ethnicity and neighborhood poverty level) on OS, NRM, and relapse after adjusting for demographic and disease variables. A significance level of 0.05 was used in our analyses.
The interaction between race/ethnicity and neighborhood poverty level was evaluated. Non-Hispanic White for race/ethnicity and low poverty level for SES were considered the referent groups for analyses. With these baseline groups, we had a sample size with the ability to detect a 10% difference in survival at 5 years among race/ethnicity groups, with an 80% power and hazard ratio of 0.65.
Variables considered for inclusion into the model: race/ethnicity and poverty (main effects which are always kept in the model), age at transplant, co-morbidity index (HCT-CI), insurance type, marital status, distance between residence and transplant center, location of residence (urban/rural), disease risk index at transplant (low/intermediate/high/very high), year of transplantation, conditioning regimen, donor/graft type, GVHD prophylaxis, development of acute GVHD by 1 year post HCT, chronic GVHD by 1 year post HCT. Center effect was accounted for via random effect with log-normal distribution.
We also conducted analyses to estimate the standardized mortality ratios (SMR) for non-Hispanic Whites, non-Hispanic Blacks, and Hispanics comparing the number of observed deaths in our cohort to expected number of deaths within their age- and sex-matched general population controls in the US. SMR could not be estimated for Asians since life tables for this racial group are not available through the National Center for Health Statistics. The SMR analysis was performed according to methods described in prior CIBMTR studies.23–26 Analyses were performed using SAS statistical software (SAS Institute, Cary, NC). All tests are two-sided, and 0.05 level of significance was used throughout the study.
RESULTS
Patient and Transplant Characteristics
The characteristics of the study population by race/ethnicity are presented in Table 1. Median follow up of survivors from 1-year post-HCT was 69 months for Non-Hispanic Whites, 50 months for Non-Hispanic Black, 59 months for Hispanics, and 57 months for Asians. There were significant differences in neighborhood poverty levels by race with a greater proportion of non-Hispanic Black (37%) and Hispanic (26%) recipients residing in high poverty areas compared to non-Hispanic Whites (10%) and Asians (10%) (P<0.01). Significantly greater proportion of non-Hispanic White patients were privately insured compared to non-Hispanic Black and Hispanic patients who were more likely to be on Medicaid. Non-Hispanic Whites were also significantly more likely to be married, have a rural residence, and live at a greater distance from the transplant center. There were significant differences in the four race/ethnicity cohorts by age at transplantation, sex, Karnofsky score at HCT, HCT-CI score at HCT, diagnosis, disease risk index, donor type, graft type, conditioning regimen intensity, use of total body irradiation (TBI), GVHD prophylaxis, and year of transplantation.
Table 1.
Characteristics of adult patients receiving first allogeneic HCT for AML, ALL, CML, or MDS between 2007–2017 in the United States, by race/ethnicity
| Characteristic | Non-Hispanic white | Non-Hispanic black | Hispanic | Asian | P-Valuea |
|---|---|---|---|---|---|
|
| |||||
| No. of patients | 4385 | 338 | 516 | 234 | |
| No. of centers | 140 | 83 | 94 | 68 | |
| Age at HCT | <.01 | ||||
| Median (min-max) | 55 (18–83) | 47 (18–75) | 41 (18–76) | 47 (18–74) | |
| 18–29 | 467 (11) | 45 (13) | 154 (30) | 42 (18) | |
| 30–39 | 469 (11) | 62 (18) | 92 (18) | 44 (19) | |
| 40–49 | 716 (16) | 88 (26) | 102 (20) | 44 (19) | |
| 50–59 | 1243 (28) | 71 (21) | 91 (18) | 48 (21) | |
| 60–69 | 1271 (29) | 67 (20) | 68 (13) | 45 (19) | |
| ≥70 | 219 (5) | 5 (1) | 9 (2) | 11 (5) | |
| Recipient sex | <.01 | ||||
| Male | 2530 (58) | 148 (44) | 283 (55) | 118 (50) | |
| Female | 1855 (42) | 190 (56) | 233 (45) | 116 (50) | |
| Neighborhood poverty level* | <.01 | ||||
| Low | 3937 (90) | 214 (63) | 383 (74) | 210 (90) | |
| High | 448 (10) | 124 (37) | 133 (26) | 24 (10) | |
| Health insurance type | <.01 | ||||
| Disability insurance +/−others | 103 (2) | 10 (3) | 8 (2) | 6 (3) | |
| Private health insurance +/− others | 2889 (66) | 181 (54) | 251 (49) | 144 (62) | |
| Medicaid +/−others | 420 (10) | 85 (25) | 171 (33) | 40 (17) | |
| Medicare +/−others | 810 (18) | 40 (12) | 58 (11) | 28 (12) | |
| Other | 120 (3) | 17 (5) | 19 (4) | 8 (3) | |
| Missing | 43 (1) | 5 (1) | 9 (2) | 8 (3) | |
| Highest level of education completed | <.01 | ||||
| No primary | 1 (0) | 0 (0) | 1 (0) | 1 (0) | |
| Less than primary | 2 (0) | 1 (0) | 4 (1) | 1 (0) | |
| Primary | 4 (0) | 0 (0) | 22 (4) | 1 (0) | |
| Lower secondary | 74 (2) | 11 (3) | 35 (7) | 4 (2) | |
| Upper secondary | 1015 (23) | 110 (33) | 164 (32) | 37 (16) | |
| Post-secondary (vocational) | 358 (8) | 37 (11) | 47 (9) | 11 (5) | |
| Tertiary (4-year degree) | 1094 (25) | 65 (19) | 50 (10) | 56 (24) | |
| Tertiary (2-year degree) | 235 (5) | 18 (5) | 26 (5) | 14 (6) | |
| Advanced research degree | 212 (5) | 13 (4) | 15 (3) | 25 (11) | |
| Missing | 1390 (32) | 83 (25) | 152 (29) | 84 (36) | |
| Marital status | <.01 | ||||
| Single, never married | 626 (14) | 96 (28) | 161 (31) | 41 (18) | |
| Married | 3163 (72) | 179 (53) | 286 (55) | 152 (65) | |
| Separated | 47 (1) | 8 (2) | 12 (2) | 4 (2) | |
| Divorced | 356 (8) | 32 (9) | 35 (7) | 12 (5) | |
| Widowed | 105 (2) | 7 (2) | 8 (2) | 8 (3) | |
| Missing | 88 (2) | 16 (5) | 14 (3) | 17 (7) | |
| Urban/rural residence | <.01 | ||||
| Urban | 3733 (85) | 316 (93) | 498 (97) | 228 (97) | |
| Rural | 652 (15) | 22 (7) | 18 (3) | 6 (3) | |
| Distance between residence and transplant center (miles) | <.01 | ||||
| < 20 | 1281 (29) | 168 (50) | 238 (46) | 116 (50) | |
| 20–50 | 1045 (24) | 52 (15) | 106 (21) | 60 (26) | |
| 50–150 | 1247 (28) | 72 (21) | 82 (16) | 26 (11) | |
| > 150 | 812 (19) | 46 (14) | 90 (17) | 32 (14) | |
| Karnofsky score at HCT | <.01 | ||||
| <90 | 1569 (36) | 133 (39) | 140 (27) | 75 (32) | |
| ≥90 | 2738 (62) | 201 (59) | 370 (72) | 155 (66) | |
| Missing | 78 (2) | 4 (1) | 6 (1) | 4 (2) | |
| HCT-CI score at HCT | <.01 | ||||
| 0 | 1078 (25) | 74 (22) | 196 (38) | 82 (35) | |
| 1–2 | 1145 (26) | 105 (31) | 128 (25) | 58 (25) | |
| ≥3 | 1719 (39) | 140 (41) | 157 (30) | 85 (36) | |
| Missing | 443 (10) | 19 (6) | 35 (7) | 9 (4) | |
| Disease | <.01 | ||||
| AML | 2225 (51) | 172 (51) | 222 (43) | 125 (53) | |
| ALL | 544 (12) | 75 (22) | 180 (35) | 44 (19) | |
| CML | 167 (4) | 21 (6) | 34 (7) | 7 (3) | |
| MDS | 1449 (33) | 70 (21) | 80 (16) | 58 (25) | |
| Refined disease risk index | <.01 | ||||
| Low | 307 (7) | 27 (8) | 56 (11) | 21 (9) | |
| Intermediate | 2347 (54) | 204 (60) | 286 (55) | 151 (65) | |
| High | 977 (22) | 68 (20) | 101 (20) | 43 (18) | |
| Very high | 60 (1) | 5 (1) | 11 (2) | 1 (0) | |
| N/A - year of transplant <2008 | 457 (10) | 18 (5) | 41 (8) | 8 (3) | |
| Missing | 237 (5) | 16 (5) | 21 (4) | 10 (4) | |
| Donor type | <.01 | ||||
| HLA-identical sibling | 1368 (31) | 101 (30) | 189 (37) | 92 (39) | |
| Other related | 357 (8) | 99 (29) | 84 (16) | 31 (13) | |
| Well-matched unrelated (8/8) | 1972 (45) | 40 (12) | 93 (18) | 46 (20) | |
| Partially-matched unrelated (7/8) | 317 (7) | 34 (10) | 56 (11) | 18 (8) | |
| Mis-matched unrelated (≤6/8) | 16 (0) | 1 (0) | 3 (1) | 3 (1) | |
| Cord blood | 355 (8) | 63 (19) | 91 (18) | 44 (19) | |
| Graft type | <.01 | ||||
| Bone marrow | 631 (14) | 49 (14) | 73 (14) | 29 (12) | |
| Peripheral blood | 3399 (78) | 226 (67) | 352 (68) | 161 (69) | |
| Cord blood | 355 (8) | 63 (19) | 91 (18) | 44 (19) | |
| Conditioning intensity | <.01 | ||||
| MAC | 2636 (60) | 232 (69) | 373 (72) | 158 (68) | |
| RIC/NMA | 1737 (40) | 105 (31) | 140 (27) | 76 (32) | |
| Missing | 12 (0) | 1 (0) | 3 (1) | 0 (0) | |
| TBI usage | <.01 | ||||
| No | 2791 (64) | 158 (47) | 248 (48) | 114 (49) | |
| Yes | 1582 (36) | 179 (53) | 265 (51) | 120 (51) | |
| Missing | 12 (0) | 1 (0) | 3 (1) | 0 (0) | |
| GVHD prophylaxis | <.01 | ||||
| Post-CY + other(s) | 307 (7) | 86 (25) | 71 (14) | 32 (14) | |
| Post-CY alone | 12 (0) | 0 (0) | 1 (0) | 0 (0) | |
| TAC + MMF +- other(s) (except post-CY) | 817 (19) | 64 (19) | 68 (13) | 20 (9) | |
| TAC + MTX +- other(s) (except MMF, post-CY) | 2149 (49) | 115 (34) | 228 (44) | 101 (43) | |
| TAC + other(s) (except MMF, MTX, post-CY) | 312 (7) | 13 (4) | 55 (11) | 25 (11) | |
| TAC alone | 110 (3) | 12 (4) | 13 (3) | 6 (3) | |
| CSA + MMF +- other(s) (except post-CY) | 368 (8) | 31 (9) | 55 (11) | 38 (16) | |
| CSA + MTX +- other(s) (except MMF, post-CY) | 211 (5) | 10 (3) | 17 (3) | 9 (4) | |
| CSA + other(s) (except MMF, MTX, post-CY) | 20 (0) | 1 (0) | 2 (0) | 0 (0) | |
| CSA alone | 16 (0) | 1 (0) | 4 (1) | 1 (0) | |
| Other(s) | 42 (1) | 1 (0) | 1 (0) | 1 (0) | |
| Missing | 21 (0) | 4 (1) | 1 (0) | 1 (0) | |
| Year of HCT | <.01 | ||||
| 2007 | 457 (10) | 18 (5) | 41 (8) | 8 (3) | |
| 2008 | 592 (14) | 36 (11) | 64 (12) | 17 (7) | |
| 2009 | 578 (13) | 30 (9) | 61 (12) | 25 (11) | |
| 2010 | 449 (10) | 39 (12) | 53 (10) | 26 (11) | |
| 2011 | 311 (7) | 17 (5) | 38 (7) | 19 (8) | |
| 2012 | 324 (7) | 19 (6) | 44 (9) | 15 (6) | |
| 2013 | 486 (11) | 29 (9) | 56 (11) | 30 (13) | |
| 2014 | 420 (10) | 27 (8) | 42 (8) | 17 (7) | |
| 2015 | 324 (7) | 44 (13) | 47 (9) | 26 (11) | |
| 2016 | 244 (6) | 45 (13) | 40 (8) | 29 (12) | |
| 2017 | 200 (5) | 34 (10) | 30 (6) | 22 (9) | |
| Acute GVHD (grade II-IV) | 0.44 | ||||
| No | 2521 (57) | 214 (63) | 290 (56) | 142 (61) | |
| Yes | 1837 (42) | 122 (36) | 223 (43) | 91 (39) | |
| Missing | 27 (1) | 2 (1) | 3 (1) | 1 (0) | |
| Chronic GVHD before 1 year | 0.76 | ||||
| No | 1904 (43) | 144 (43) | 219 (42) | 102 (44) | |
| Yes | 2447 (56) | 193 (57) | 290 (56) | 130 (56) | |
| Missing | 34 (1) | 1 (0) | 7 (1) | 2 (1) | |
| Follow-up of survivors from 1 year post-HCT (months) - median (range) | 69 (0–138) | 50 (0–133) | 59 (0–133) | 57 (0–130) | |
HCT – hematopoietic cell transplantation; HCT-CI – hematopoietic cell transplantation comorbidity index; AML – acute myeloid leukemia; ALL – acute lymphoblastic leukemia; CML – chronic myeloid leukemia; MDS – myelodysplastic syndrome; MAC – myeloablative conditioning; RIC/NMA – reduced intensity/non-myeloablative conditioning; TBI – total body irradiation; Cy – cyclophosphamide; MMF – mycophenolate mofetil; TAC – tacrolimus; CSA – cyclosporine; MTX – methotrexate; GVHD – graft-versus-host disease
High poverty neighborhood defined as ≥20% of persons living below 100% federal poverty level; low-poverty neighborhood defined as <20% of persons below 100% federal poverty level Hypothesis testing:
Pearson chi-square test
Table 2 shows our cohorts characteristics by neighborhood poverty level. Median follow up of survivors for both low and high poverty areas was 62 months, respectively. As noted above, a significantly greater proportion of non-Hispanic Blacks and Hispanics live in high poverty neighborhoods. As expected, a greater number of recipients residing in low poverty neighborhoods were significantly more likely to have private health insurance, be married, have an urban residence, and lived closer to the transplant center. Among disease and transplant related characteristics, significant differences between the two cohorts were noted in age at transplantation, diagnosis, and donor type.
Table 2.
Characteristics of adult patients receiving first allogeneic HCT for AML, ALL, CML, MDS between 2007–2017 in the United States, by neighborhood poverty level*
| Characteristic | Low poverty | High poverty P | Valuea |
|---|---|---|---|
|
| |||
| No. of patients | 4744 | 729 | |
| No. of centers | 142 | 106 | |
| Age at HCT | <.01 | ||
| Median (min-max) | 54 (18–83) | 50 (18–76) | |
| 18–29 | 585 (12) | 123 (17) | |
| 30–39 | 562 (12) | 105 (14) | |
| 40–49 | 808 (17) | 142 (19) | |
| 50–59 | 1285 (27) | 168 (23) | |
| 60–69 | 1281 (27) | 170 (23) | |
| ≥70 | 223 (5) | 21 (3) | |
| Recipient sex | 0.15 | ||
| Male | 2687 (57) | 392 (54) | |
| Female | 2057 (43) | 337 (46) | |
| Race | <.01 | ||
| Non-Hispanic white | 3937 (83) | 448 (61) | |
| Non-Hispanic black | 214 (5) | 124 (17) | |
| Hispanic | 383 (8) | 133 (18) | |
| Asian | 210 (4) | 24 (3) | |
| Health insurance type | <.01 | ||
| Disability insurance +/−others | 114 (2) | 13 (2) | |
| Private health insurance +/− others | 3076 (65) | 389 (53) | |
| Medicaid +/−others | 533 (11) | 183 (25) | |
| Medicare +/−others | 820 (17) | 116 (16) | |
| Other | 139 (3) | 25 (3) | |
| Missing | 62 (1) | 3 (0) | |
| Highest level of education completed | <.01 | ||
| No primary | 3 (0) | 0 (0) | |
| Less than primary | 6 (0) | 2 (0) | |
| Primary | 17 (0) | 10 (1) | |
| Lower secondary | 95 (2) | 29 (4) | |
| Upper secondary | 1122 (24) | 204 (28) | |
| Post-secondary (vocational) | 391 (8) | 62 (9) | |
| Tertiary (4-year degree) | 1138 (24) | 127 (17) | |
| Tertiary (2-year degree) | 258 (5) | 35 (5) | |
| Advanced research degree | 234 (5) | 31 (4) | |
| Missing | 1480 (31) | 229 (31) | |
| Marital status | <.01 | ||
| Single, never married | 752 (16) | 172 (24) | |
| Married | 3347 (71) | 433 (59) | |
| Separated | 50 (1) | 21 (3) | |
| Divorced | 368 (8) | 67 (9) | |
| Widowed | 106 (2) | 22 (3) | |
| Missing | 121 (3) | 14 (2) | |
| Urban/rural residence | <.01 | ||
| Urban | 4190 (88) | 585 (80) | |
| Rural | 554 (12) | 144 (20) | |
| Distance between residence and transplant center (miles) | <.01 | ||
| < 20 | 1574 (33) | 229 (31) | |
| 20–50 | 1181 (25) | 82 (11) | |
| 50–150 | 1187 (25) | 240 (33) | |
| >150 | 802 (17) | 178 (24) | |
| Karnofsky score | 0.92 | ||
| <90 | 1666 (35) | 251 (34) | |
| ≥90 | 2999 (63) | 465 (64) | |
| Missing | 79 (2) | 13 (2) | |
| HCT-CI | 0.34 | ||
| 0 | 1232 (26) | 198 (27) | |
| 1–2 | 1263 (27) | 173 (24) | |
| ≥3 | 1818 (38) | 283 (39) | |
| Missing | 431 (9) | 75 (10) | |
| Disease | <.01 | ||
| AML | 2371 (50) | 373 (51) | |
| ALL | 700 (15) | 143 (20) | |
| CML | 192 (4) | 37 (5) | |
| MDS | 1481 (31) | 176 (24) | |
| Refined disease risk index | 0.54 | ||
| Low | 358 (8) | 53 (7) | |
| Intermediate | 2582 (54) | 406 (56) | |
| High | 1043 (22) | 146 (20) | |
| Very high | 68 (1) | 9 (1) | |
| N/A - year of transplant <2008 | 443 (9) | 81 (11) | |
| Missing | 250 (5) | 34 (5) | |
| Donor type | <.01 | ||
| HLA-identical sibling | 1515 (32) | 235 (32) | |
| Other related | 482 (10) | 89 (12) | |
| Well-matched unrelated (8/8) | 1913 (40) | 238 (33) | |
| Partially-matched unrelated (7/8) | 350 (7) | 75 (10) | |
| Mis-matched unrelated (≤6/8) | 19 (0) | 4 (1) | |
| Cord blood | 465 (10) | 88 (12) | |
| Graft type | 0.10 | ||
| Bone marrow | 671 (14) | 111 (15) | |
| Peripheral blood | 3608 (76) | 530 (73) | |
| Cord blood | 465 (10) | 88 (12) | |
| Conditioning intensity | 0.75 | ||
| MAC | 2937 (62) | 462 (63) | |
| RIC/NMA | 1793 (38) | 265 (36) | |
| Missing | 14 (0) | 2 (0) | |
| TBI usage | 0.80 | ||
| No | 2878 (61) | 433 (59) | |
| Yes | 1852 (39) | 294 (40) | |
| Missing | 14 (0) | 2 (0) | |
| GVHD prophylaxis | 0.83 | ||
| Post-CY + other(s) | 417 (9) | 79 (11) | |
| Post-CY alone | 12 (0) | 1 (0) | |
| TAC + MMF +- other(s) (except post-CY) | 827 (17) | 142 (19) | |
| TAC + MTX +- other(s) (except MMF, post-CY) | 2261 (48) | 332 (46) | |
| TAC + other(s) (except MMF, MTX, post-CY) | 354 (7) | 51 (7) | |
| TAC alone | 124 (3) | 17 (2) | |
| CSA + MMF +- other(s) (except post-CY) | 433 (9) | 59 (8) | |
| CSA + MTX +- other(s) (except MMF, post-CY) | 214 (5) | 33 (5) | |
| CSA + other(s) (except MMF, MTX, post-CY) | 20 (0) | 3 (0) | |
| CSA alone | 19 (0) | 3 (0) | |
| Other(s) | 39 (1) | 6 (1) | |
| Missing | 24 (1) | 3 (0) | |
| Year of HCT | 0.25a | ||
| 2007 | 443 (9) | 81 (11) | |
| 2008 | 600 (13) | 109 (15) | |
| 2009 | 617 (13) | 77 (11) | |
| 2010 | 496 (10) | 71 (10) | |
| 2011 | 340 (7) | 45 (6) | |
| 2012 | 350 (7) | 52 (7) | |
| 2013 | 529 (11) | 72 (10) | |
| 2014 | 440 (9) | 66 (9) | |
| 2015 | 372 (8) | 69 (9) | |
| 2016 | 313 (7) | 45 (6) | |
| 2017 | 244 (5) | 42 (6) | |
| Acute GVHD (grade II-IV) | 0.19a | ||
| No | 2727 (57) | 440 (60) | |
| Yes | 1986 (42) | 287 (39) | |
| Missing | 31 (1) | 2 (0) | |
| Chronic GVHD before 1 year | 0.74 | ||
| No | 2044 (43) | 325 (45) | |
| Yes | 2662 (56) | 398 (55) | |
| Missing | 38 (1) | 6 (1) | |
| Follow-up of survivors from 1 year post-HCT (months)- median (range) | 62 (0–138) | 62 (0–134) | |
HCT – hematopoietic cell transplantation; HCT-CI – hematopoietic cell transplantation comorbidity index; AML – acute myeloid leukemia; ALL – acute lymphoblastic leukemia; CML – chronic myeloid leukemia; MDS – myelodysplastic syndrome; MAC – myeloablative conditioning; RIC/NMA – reduced intensity/non-myeloablative conditioning; TBI – total body irradiation; Cy – cyclophosphamide; MMF – mycophenolate mofetil; TAC – tacrolimus; CSA – cyclosporine; MTX – methotrexate; GVHD – graft-versus-host disease
High poverty neighborhood defined as ≥20% of persons living below 100% federal poverty level; low-poverty neighborhood defined as <20% of persons below 100% federal poverty level
Hypothesis testing:
Pearson chi-square test
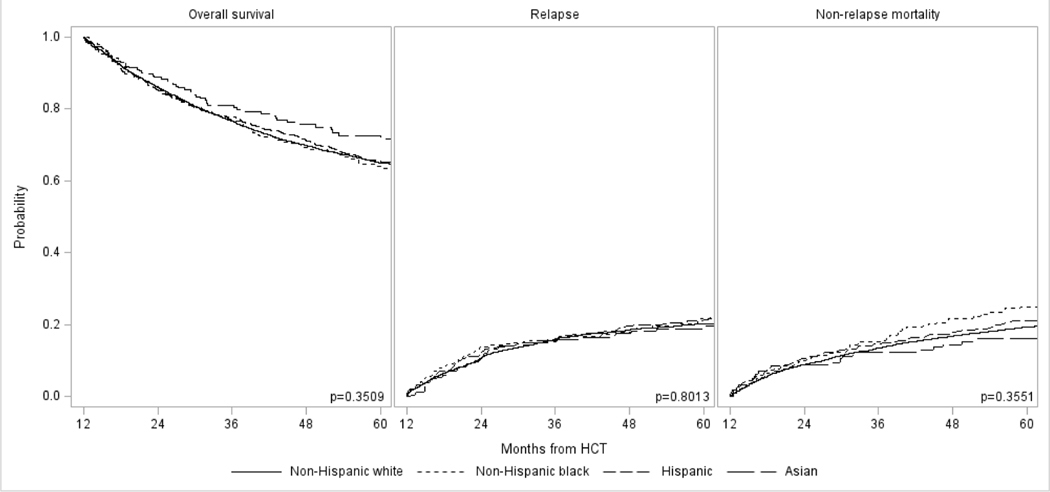
Analysis by Race/Ethnicity
Table 3 highlights outcomes from the 1-year post-transplant time point. There was a significant difference in OS at 5 years with highest survival in Asian recipients (74%), followed by Hispanic (70%), and non-Hispanic White (65%) and non-Hispanic Black (65%) patients (P=0.003). There was no difference in the cumulative incidence of relapse, although the cumulative incidence of NRM mirrored that of OS with lowest rates in Asians (13%), followed by Hispanics (16%), and non-Hispanic Blacks (20%) and non-Hispanic Whites (21%) (P=0.002). However, in multivariable analyses adjusting for transplant, disease, and transplant related characteristics, there was no difference in OS or NRM between the four race/ethnicity groups (Table 4). Of note, there was a significant association noted between urban/rural place of residence and insurance status and OS and NRM. Recipients with rural residence have higher risks of overall mortality (hazard ratio [HR] 1.22 vs. urban residence, 95% CI 1.08–1.38; P=0.002) and NRM (HR 1.33, 95% CI 1.13–1.57; P=0.0005). Compared to patients with private health insurance, higher risk of overall mortality was seen in patients on Medicaid (HR 1.17, 95% CI 1.01–1.35; P=0.033) and on Medicare (HR 1.22, 95% CI 1.07–1.39, P=0.0029), although this association was only observed in Medicare recipients for NRM (HR 1.25, 95% CI 1.06–1.48; P=0.008). There was no association between residence location and insurance status with risks of relapse. The causes of death were similar in all four groups, with disease relapse being the most common cause followed by GVHD, infections, and organ failure.
Table 3.
Univariate analysis for 5-year outcomes, by race/ethnicity and neighborhood poverty level
| Overall survival | Relapse | Non-relapse mortality | |||||
|---|---|---|---|---|---|---|---|
| Outcomes# | N | Probability (95% CI) | P-value | Probability (95% CI) | P-value | Probability (95% CI) | P-value |
|
| |||||||
| Race/Ethnicity | |||||||
| Non-Hispanic White | 4385 | 65 (64–66)% | 0.003 | 20 (19–21)% | 0.218 | 21 (19–22)% | 0.002 |
| Non-Hispanic Black | 338 | 65 (59–70)% | 23 (19–28)% | 20 (15–25)% | |||
| Hispanic | 516 | 70 (65–74)% | 21 (17–25)% | 16 (13–20)% | |||
| Asian | 234 | 74 (67–80)% | 19 (14–25)% | 13 (9–18)% | |||
| Neighborhood poverty level* | |||||||
| Low | 4744 | 66 (65–68)% | 0.156 | 20 (18–21)% | 0.108 | 20 (19–21)% | 0.653 |
| High | 729 | 62 (58–66)% | 23 (20–26)% | 20 (17–23)% | |||
CI – confidence intervals
Outcome estimates are from 1-year post-transplant
High poverty neighborhood defined as ≥20% of persons living below 100% federal poverty level; low-poverty neighborhood defined as <20% of persons below 100% federal poverty level
Table 4.
Results of multivariable analysis
| Overall survivala | Relapseb | Non-relapse mortalityc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | N | Hazard ratio (95% CI) | P-value | N | Hazard ratio (95% CI) | P-value | N | Hazard ratio (95% CI) | P-value |
|
| |||||||||
| Race/Ethnicity | |||||||||
| Non-Hispanic White | 4385 | 1.00 | 0.3509 | 4385 | 1.00 | 0.8013 | 4373 | 1.00 | 0.3551 |
| Non-Hispanic Black | 338 | 1.16 (0.95–1.41) | 0.1433 | 338 | 1.10 (0.86–1.41) | 0.4418 | 338 | 1.24 (0.95–1.62) | 0.1226 |
| Hispanic | 516 | 1.01 (0.84–1.20) | 0.9500 | 516 | 1.05 (0.85–1.30) | 0.6601 | 515 | 1.04 (0.82–1.32) | 0.7570 |
| Asian | 234 | 0.89 (0.69–1.15) | 0.3636 | 234 | 0.94 (0.69–1.27) | 0.6818 | 234 | 0.87 (0.61–1.25) | 0.4488 |
| Neighborhood poverty level* | |||||||||
| Low | 4744 | 1.00 | 0.1184 | 4744 | 1.00 | 0.1251 | 4732 | 1.00 | 0.8299 |
| High | 729 | 1.11 (0.97–1.26) | 729 | 1.14 (0.96–1.36) | 728 | 1.02 (0.86–1.21) | |||
CI – confidence intervals
High poverty neighborhood defined as ≥20% of persons living below 100% federal poverty level; low-poverty neighborhood defined as <20% of persons below 100% federal poverty level
Other variables significantly associated with overall survival included age at HCT, sex, acute graft-versus-host disease, chronic graft-versus-host disease by 1-year post-transplant, conditioning regimen intensity, donor type, disease risk index, HCT comorbidity index score, insurance type, rural/urban residence, and year of HCT
Other variables significantly associated with relapse included acute graft-versus-host disease, chronic graft-versus-host disease by 1-year post-transplant, conditioning regimen intensity, donor type, disease risk index, and year of HCT
Other variables significantly associated with non-relapse mortality included age at HCT, sex, acute graft-versus-host disease, chronic graft-versus-host disease by 1-year post-transplant, donor type, graft type, HCT comorbidity index score, graft-versus-host disease prophylaxis, insurance type, rural/urban residence, and year of HCT (13 patients who had received post-transplant cyclophosphamide as graft-versus-host disease prophylaxis were excluded from the multivariable analysis for non-relapse mortality since they did not have any events)
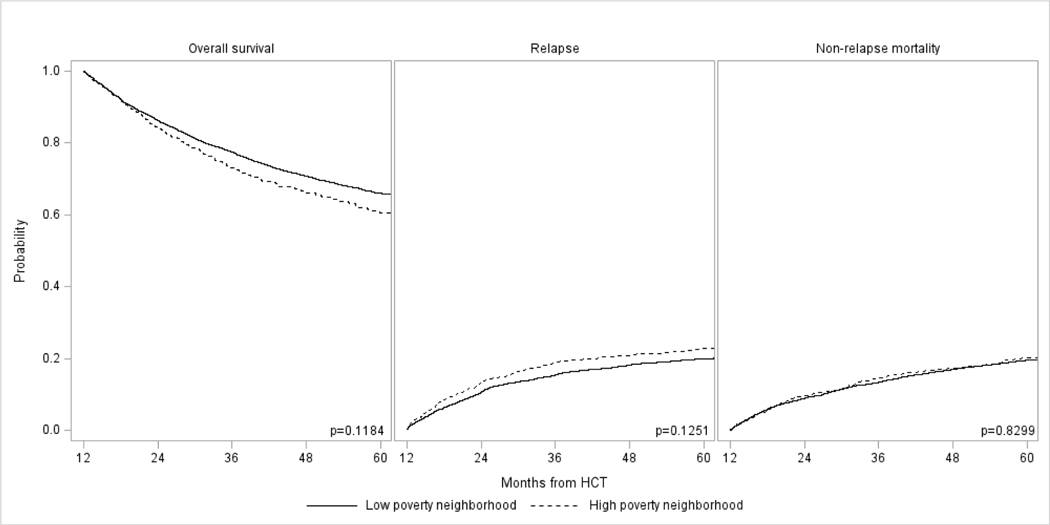
Analysis by Neighborhood Poverty Level
Tables 3 and 4 also show results for univariate and multivariable analysis by neighborhood poverty level. In univariate analysis, there was no difference in 5-year OS, relapse, or NRM between patients coming from low and high poverty neighborhoods. This was confirmed in multivariable analyses, which again did not show any significant differences in risks of OS, relapse, or NRM among the two groups. As noted above, residence location and insurance status were associated with OS and NRM.
Mortality Rates Compared to General Population
The analysis comparing observed versus expected mortality for non-Hispanic White, non-Hispanic Black, and Hispanic recipients is shown in Table 5. Our cohort of 1-year HCT survivors had mortality rates that were approximately 10-times higher than their age- and sex-matched controls from the general population. We also observed differences by race/ethnicity, with Hispanic recipients having higher mortality rates (SMR 19.2) than non-Hispanic Whites (SMR 10.0) and non-Hispanic Blacks (SMR 11.2).
Table 5.
Standardized mortality ratios for non-Hispanic White, non-Hispanic Black, and Hispanic patients compared to their age- and sex-matched general population controls
| Population | N | Person years | Observed | Expected | SMR (95% CI) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| All patients | 5239 | 23346 | 2074 | 198.6 | 10.4 (10.0–10.9) | <0.0001 |
| Non-Hispanic White | 4385 | 19950 | 1791 | 179.2 | 10.0 (9.5–10.5) | <0.0001 |
| Non-Hispanic Black | 338 | 1254 | 124 | 11.1 | 11.2 (9.3–13.3) | <0.0001 |
| Hispanic | 516 | 2142 | 159 | 8.3 | 19.2 (16.4–22.5) | <0.0001 |
CI – confidence intervals
DISCUSSION
In our study of adult allogeneic HCT recipients with hematologic malignancies who had survived in remission for ≥1 year, we observed no differences in OS, relapse, or NRM either by race/ethnicity or by neighborhood poverty levels. Previous CIBMTR studies that have considered HCT recipients from the time of transplantation have demonstrated significant differences in survival by race/ethnicity, neighborhood poverty levels, and community health status1,3,5. We had hypothesized that these disparities would persist or even be accentuated in long-term survivors since many patients frequently transition back to their local healthcare ecosystem after the first 3–6 months following transplantation, where the systemic racial and socioeconomic disparity factors may be prevalent in their communities. Previously recognized disparity in the outcomes of allogeneic HCT is once again evidenced by the differences in overall survival and non-relapse mortality seen in this study. However, these differences in OS and NRM among various racial/ethnic groups become less pronounced when adjusted for other risk factors. These findings may suggest that those differences are occurring due to the variability in certain patient and treatment characteristics of different racial/ethnic groups.
We postulate several hypotheses for our observed lack of association between race/ethnicity, neighborhood poverty level, and outcomes in long-term HCT survivors seen after accounting for other risk factors and sociodemographic characteristics. First, patients from minority groups who are able to receive allogeneic transplantation are not representative of the universe of racial/ethnic and socioeconomically disadvantaged populations. For example, HCT recipients have been reported to have higher literacy levels, education status, and SES than the general population8,27,28. Hence, HCT survivors who transition back to their communities may have different and better healthcare access and experience than their peers in the general population. Second, a substantial portion of mortality occurs in the first year following allogeneic transplantation, where the impact of healthcare disparities may be the most significant. Also, a larger proportion of racial minority patients lived closer to their transplant center and may have had the advantage of closer follow up by their treating institution. For example, among non-Hispanic Whites, 53% lived within 50 miles of the transplant center and 15% reported a rural place of residence. In contrast, a greater proportion of racial/ethnic minority recipients lived close to the transplant center (65% non-Hispanic Blacks, 66% Hispanics, 66% Asians lived within 50 miles) or in an urban area (7%, 3%, and 3%, respectively, reported rural residence). It is also important to note that our study cohort consisted of patients transplanted in the contemporary era where there is emphasis on providing systematic post-transplant survivorship care, routine utilization of social workers to assess and provide psychosocial support to patients and their families, and increased availability of financial and other support services from patient advocacy organizations. These factors may have mitigated the adverse effect of healthcare disparity factors in long-term HCT survivors. Additionally, the use of newer GVHD prevention strategies such as post-transplant cyclophosphamide may have contributed, as evidenced by comparable rates of acute and chronic GVHD among the four race groups despite the greater use of donors other than HLA-identical siblings or matched unrelated donors in racial minorities.
In a related single center study of allogeneic and autologous HCT ≥1 year survivors, Joo et al also did not find any association between post-transplant survival and community health status – a measure that was previously reported to significantly influence 1-year survival3,29. It is well recognized that healthcare disparities is a complex construct, and as supported by our analysis, other related factors such as insurance status and neighborhood poverty level may be the mechanism for adverse outcomes previously reported among minority HCT recipients.
An important finding from our study that has immediate application in clinical practice is the observation of mortality risks that are ~10 times higher for non-Hispanic Whites and non-Hispanic Blacks and ~20 times higher for Hispanics. This is consistent with other studies that have shown higher rates of mortality in allogeneic HCT survivors than what may be expected in the general population till at least 10–15 years post-transplantation24,30–32. Our study did show worse mortality for the Hispanic population, we postulate this is due to health-related factors associated with decreased survival other than the primary malignancy. More work is needed to understand why this minority community continues to show worsening mortality. Our findings underscore the importance of continued lifelong vigilance for late complications of transplantation with screening, prevention, and prompt management according to published guidelines14,15,17. There is additionally a need to develop care models and infrastructure such as survivorship care plans, telemedicine, web based self-management applications, that can facilitate the care of long-term survivors who may not be under the direct care of their transplant centers27,33–35.
Some limitations of our study have to be considered. First, our study is limited due to its retrospective registry-based design. As noted earlier, we cannot address the totality of healthcare disparities in transplantation given that our study population consisted of patients who were able to get to an allogeneic HCT and we have no mechanism of ascertaining patients who needed a transplant but could not receive one. It is also possible that we are underestimating the true representation of long-term care for HCT survivors. Although we had a very robust median follow up it is still possible that longer follow up may be needed to reveal differences in late effects that manifest much later post-transplant. Our findings are only applicable to the US and are not generalizable to other countries.
In summary, we found that among adult allogeneic HCT recipients who survived for at least 1 year in remission, there were no significant differences in OS, relapse, or NRM based on race/ethnicity or neighborhood poverty level. HCT survivors have significantly higher risks of mortality compared to the general population and require long-term monitoring and prevention of late effects irrespective of race/ethnicity or SES.
Supplementary Material
Figure 1:
Long-term outcomes in adult allogeneic HCT survivors with AML, ALL, CML, and MDS who had survived in remission ≥1 year in remission by race/ethnicity: (A) Overall survival, (B) Relapse, and (C) Non-relapse mortality
Figure 2:
Long-term outcomes in adult allogeneic HCT survivors with AML, ALL, CML, and MDS who had survived in remission ≥1 year in remission by neighborhood poverty level: (A) Overall survival, (B) Relapse, and (C) Non-relapse mortality
Acknowledgments
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Gilead; GlaxoSmithKline; Incyte Corporation; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karyopharm Therapeutics; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Medac GmbH; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncopeptides, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Footnotes
Data Analysis and Interpretation: All Authors
Financial Disclosure Statement
B.Blue: Consultancy: Janssen, Pfizer, Abbvie, Oncopeptides. W. Wood: Research Funding: Pfizer; Consultancy: Tedadoc; Consultancy and Current equity holder in publicly traded company: Koneksa Health. N. Majhail: Consultancy: Incyte Corporation, Anthem, Inc. W. Saber: Other: Govt. COI. All other authors reported no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bona K, Brazauskas R, He N, et al. : Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood 137:556–568, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong S, Majhail NS: Increasing access to allotransplants in the United States: the impact of race, geography, and socioeconomics. Hematology Am Soc Hematol Educ Program 2021:275–280, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong S, Brazauskas R, Hebert KM, et al. : Community health status and outcomes after allogeneic hematopoietic cell transplantation in the United States. Cancer 127:609–618, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morishima Y, Morishima S, Stevenson P, et al. : Race and Survival in Unrelated Hematopoietic Cell Transplantation. Transplant Cell Ther 28:357 e1–357 e6, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker KS, Davies SM, Majhail NS, et al. : Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 15:1543–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell JM, Meehan KR, Kong J, et al. : Access to bone marrow transplantation for leukemia and lymphoma: the role of sociodemographic factors. J Clin Oncol 15:2644–51, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Flannelly C, Tan BE, Tan JL, et al. : Barriers to Hematopoietic Cell Transplantation for Adults in the United States: A Systematic Review with a Focus on Age. Biol Blood Marrow Transplant 26:2335–2345, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majhail NS, Omondi NA, Denzen E, et al. : Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 16:1070–5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson K, Brazauskas R, Khera N, et al. : Inferior Access to Allogeneic Transplant in Disadvantaged Populations: A Center for International Blood and Marrow Transplant Research Analysis. Biol Blood Marrow Transplant 25:2086–2090, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabo B, Morgan JW, Martinez ME, et al. : Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One 12:e0174760, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshua TV, Rizzo JD, Zhang MJ, et al. : Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer 116:3469–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt VR, Chen B, Gyawali B, et al. : Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplant 53:1288–1294, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Buchsel PC: Survivorship issues in hematopoietic stem cell transplantation. Semin Oncol Nurs 25:159–69, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, Rizzo JD, Lee SJ, et al. : Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant 47:337–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majhail NS, Rizzo JD, Lee SJ, et al. : Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 18:348–71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashmi SK, Lee SJ, Savani BN, et al. : ASBMT Practice Guidelines Committee Survey on Long-Term Follow-Up Clinics for Hematopoietic Cell Transplant Survivors. Biol Blood Marrow Transplant 24:1119–1124, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Majhail NS: Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther 10:220–227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battiwalla M, Tichelli A, Majhail NS: Long-Term Survivorship after Hematopoietic Cell Transplantation: Roadmap for Research and Care. Biol Blood Marrow Transplant 23:184–192, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin PJ, Counts GW Jr., Appelbaum FR, et al. : Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol 28:1011–6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielcarek M, Gooley T, Martin PJ, et al. : Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant 11:231–9, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Brown-Iannuzzi JL, Payne BK, Rini C, et al. : Objective and subjective socioeconomic status and health symptoms in patients following hematopoietic stem cell transplantation. Psychooncology 23:740–8, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Fu S, Rybicki L, Abounader D, et al. : Association of socioeconomic status with long-term outcomes in 1-year survivors of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 50:1326–30, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Myers RM, Hill BT, Shaw BE, et al. : Long-term outcomes among 2-year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large b-cell lymphoma. Cancer 124:816–825, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wingard JR, Majhail NS, Brazauskas R, et al. : Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29:2230–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majhail NS, Bajorunaite R, Lazarus HM, et al. : Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol 147:129–39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majhail NS, Bajorunaite R, Lazarus HM, et al. : High probability of long-term survival in 2-year survivors of autologous hematopoietic cell transplantation for AML in first or second CR. Bone Marrow Transplant 46:385–92, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majhail NS, Murphy E, Laud P, et al. : Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica 104:1084–1092, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denzen EM, Thao V, Hahn T, et al. : Financial impact of allogeneic hematopoietic cell transplantation on patients and families over 2 years: results from a multicenter pilot study. Bone Marrow Transplant 51:1233–40, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo JH, Hong S, Rybicki LA, et al. : Community health status and long-term outcomes in 1-year survivors of autologous and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 57:671–673, 2022 [DOI] [PubMed] [Google Scholar]
- 30.Bhatia S, Francisco L, Carter A, et al. : Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood 110:3784–92, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong FL, Teh JB, Atencio L, et al. : Conditional Survival, Cause-Specific Mortality, and Risk Factors of Late Mortality After Allogeneic Hematopoietic Cell Transplantation. J Natl Cancer Inst 112:1153–1161, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatia S, Dai C, Landier W, et al. : Trends in Late Mortality and Life Expectancy After Allogeneic Blood or Marrow Transplantation Over 4 Decades: A Blood or Marrow Transplant Survivor Study Report. JAMA Oncol 7:1626–1634, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preussler JM, Denzen EM, Majhail NS, et al. : Engaging hematopoietic cell transplantation patients and caregivers in the design of print and mobile application individualized survivorship care plan tools. Support Care Cancer 28:2805–2816, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denzen EM, Preussler JM, Murphy EA, et al. : Tailoring a Survivorship Care Plan: Patient and Provider Preferences for Recipients of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 25:562–569, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syrjala KL, Yi JC, Artherholt SB, et al. : An online randomized controlled trial, with or without problem-solving treatment, for long-term cancer survivors after hematopoietic cell transplantation. J Cancer Surviv 12:560–570, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.