Abstract
Spinal arachnoid web (AW) is a rare condition causing spinal cord-related issues. Its cause is often idiopathic but can be linked to past trauma or spine surgery. We describe two cases of AWs that developed after subarachnoid hemorrhage (SAH). Case #1 is a 71-year-old male with nonaneurysmal SAH who developed myelopathy 1 year later. Magnetic resonance imaging revealed upper thoracic cord edema and an AW. Case #2 is a 57-year-old female who underwent coiling of a ruptured basilar artery aneurysm and ventriculoperitoneal shunting for hydrocephalus. Twenty months later, she developed mid-thoracic AW requiring surgical resection. Both patients showed symptom improvement postresection avoiding further reoperation. History of SAH is emerging as a risk factor for AW development, emphasizing the importance of monitoring delayed-onset myelopathy and back pain in recent SAH patients.
Keywords: Arachnoid web, arachnoiditis, intradural, spinal cord, subarachnoid hemorrhage
Introduction
Clearing mechanisms and delayed complications after subarachnoid hemorrhage (SAH) or other types of brain hemorrhage are complex and not fully understood.[1,2,3] Arachnoid webs (AWs) are rare with only several dozen cases documented by 2023.[4] AWs are comprised of strands of pial-arachnoid adhesions and sometimes arachnoid cysts (AC) that can obstruct cerebrospinal fluid (CSF) flow, cause cord edema, syrinx, and result in myelopathy. Magnetic resonance imaging (MRI) often reveals cord edema without osteoligamentous compression as well as ACs. AWs typically affect otherwise healthy individuals who present with insidious-onset back pain, myelopathy, and lower-extremity weakness.
The etiology and risk factors of AWs remain largely unknown. Most cases are idiopathic, with few reports linking AWs to previous traumatic spinal injury or spine surgery.[3,4,5,6] Recently, a few case reports have linked AWs to prior SAH.[7,8,9] We present two cases of AW among 398 patients who underwent treatment for intracranial SAH at MedStar Washington Hospital Center between March 2017 and October 2022. These cases required surgical resection of the AW.
Cases
Case 1
A 71-year-old male presented with pancreatitis and associated tremors. Past medical history included chronic obstructive pulmonary disease, hypertension, and acquired immunodeficiency syndrome. Computed tomography (CT) of the head and MRI of the brain revealed scant supratentorial and infratentorial SAH [Figure 1]. MRI of the whole spine revealed diffuse SAH and a small cystic lesion at the ventral aspect of the spinal cord near the T4 vertebra [Figures 2 and 3]. Lumbar puncture was notable for xanthochromic CSF. Magnetic resonance angiogram of the brain was unremarkable. A cerebral angiogram was not performed because the patient left against medical advice and was lost to follow-up.
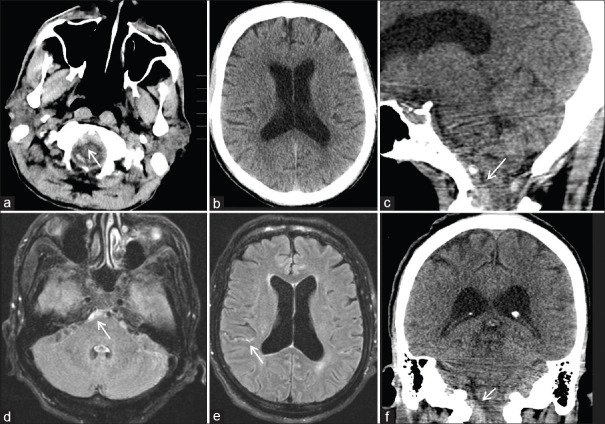
Figure 1.
Cranial imaging for Case #1 1 year before arachnoid web presentation, demonstrating the location of the subarachnoid hemorrhage (SAH). Axial computed tomography (CT) head demonstrates evidence of retroclival SAH noted with arrow (a and b). Sagittal and coronal reconstructions of the CT head redemonstrate SAH noted with arrows (c and f). Axial reconstructions of fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) of the brain demonstrate evidence of retroclival as well as supratentorial SAH, both noted with arrows (d and e)
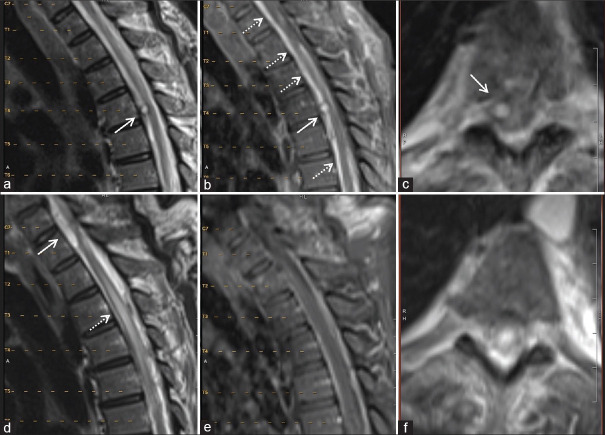
Figure 2.
Imaging of the thoracic spine for Case #1 1 year before the arachnoid web (AW) presentation (a-c) and at the time of AW (d-f). T2-weighted (a) and T1 postcontrast (b) magnetic resonance imagings (MRIs) demonstrate an intradural, extramedullary enhancing lesion (solid arrows) at the level of T4 vertebra, as well as diffuse subarachnoid enhancement likely from subarachnoid blood (dashed arrow). Axial reconstruction of T2 MRI redemonstrates the intradural extramedullary cyst on the right side of the spinal cord as noted by arrow (c). MRI obtained at the time of the AW presentation demonstrates the resolution of the lesion at the T4 vertebra, and a new intradural extramedullary nonenhancing cystic mass at the level of C7-T1 ventral to the spinal cord (noted by arrows) causing spinal cord compression and cord edema (d-f). Axial T2 MRI at the time of AW presentation reveals complete resolution of the formerly seen lesion at the T4 vertebral level
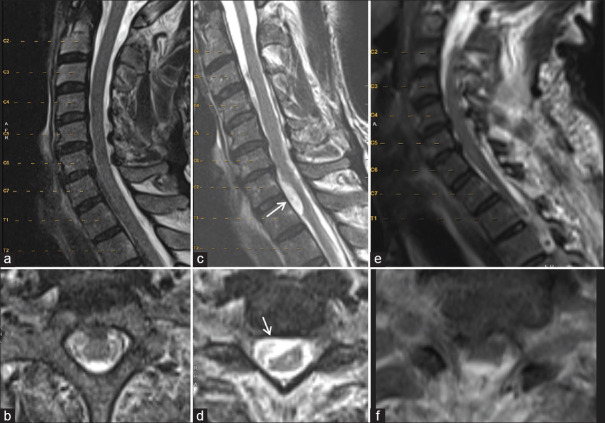
Figure 3.
Cervical spine magnetic resonance imaging (MRI) for Case #1 1 year before (a and b), at the time of arachnoid web (AW) presentation (c and d), and after the AW resection (e and f). Axial reconstructions (b, d, and f) represent the cervicothoracic junctions of respective MRIs. Note the de novo development of the ventral arachnoid cyst noted with arrows (c and d) and its resolution postoperatively (e and f)
One year later, he presented with 2 weeks of progressively worsening diffuse low back pain and difficulty ambulating. His neurological examination was notable for motor strength of 4+ in all lower-extremity muscle groups, without hyperreflexia or clonus. MRI revealed thoracic cord edema, AC, and dorsal pia-arachnoid adhesions [Figures 2 and 3]. The T4 nodular lesion that was found on the MRI from the year prior had disappeared. Diagnostic cerebral and spinal angiograms were unremarkable.
The patient underwent C7-T2 laminoplasty, and after reflection of the dural leaflets, thick arachnoid adhesions were found dorsally and were resected away from the spinal cord. Subsequently, the ventral AC was fenestrated. This resulted in the restoration of normal CSF flow in the spinal cord [Figure 3]. At 1-month and 1-year follow-ups, his back pain and problems with ambulation had resolved.
Case 2
A 57-year-old female with a history of hypertension, hyperlipidemia, and obesity presented with Hunt–Hess Grade III and Fisher Grade IV SAH and communicating hydrocephalus. A cerebral angiogram revealed a basilar trunk aneurysm, which was coiled. Ultimately, she underwent placement of a ventriculoperitoneal shunt [Figure 4]. She recovered to her neurologic baseline 4 months postprocedurally.
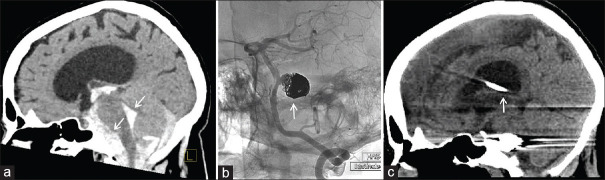
Figure 4.
Representative imaging for Case #2 2 years before arachnoid web presentation, demonstrating subarachnoid hemorrhage in the prepontine cistern and fourth ventricle (noted with arrows) with associated hydrocephalus (a). Angiogram of the vertebral artery demonstrates the appearance of a large, coiled basilar artery aneurysm (solid arrow) (b). Sagittal computed tomography head demonstrating placement of a ventriculoperitoneal shunt (white arrow) with interval resolution of the hydrocephalus (c)
Twenty months after the SAH, she was evaluated for progressive upper back pain, numbness below the umbilicus, and difficulty ambulating. Her motor strength in all lower-extremity muscle groups was 4 out of 5, and she had diffusely diminished proprioception, hyperreflexia at the patellar and Achilles tendons, and sustained clonus. MRI revealed subdural multiloculated collections as well as a “scalpel” sign at T8 [Figure 5]. She underwent T1-T6 laminoplasty for intradural exploration, and thick arachnoid adhesions were noted at the dorsal aspect of the spinal cord with no evidence of CSF flow. In addition, a hemosiderin stain was observed on the arachnoid membrane, suggesting the spread of the prior SAH into the spinal subarachnoid space. After the resection of thick arachnoid membranes and marsupialization of the AC, the spinal cord became pulsatile, and normal CSF flow was reestablished [Figure 6]. In 2 weeks, her back pain and leg numbness had resolved, and at her 5-month follow-up, she had complete resolution of symptoms.
Figure 5.
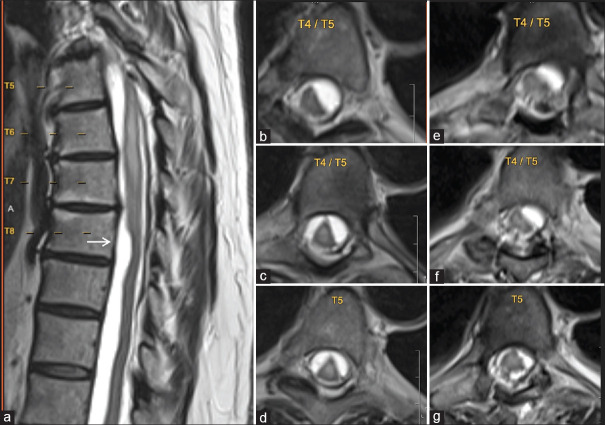
Representative magnetic resonance imaging (MRI) for Case #2 at the time of arachnoid web (AW) presentation (a-d) and postoperatively (e-g). A sagittal MRI of the thoracic spine demonstrates a radiographic finding of a “scalpel sign” at the ventral aspect of the spinal cord at the level of the T8 vertebra (solid arrow) (a). Axial reconstructions of T2-weighted MRI at various levels of the thoracic spine demonstrate the significant intradural loculations preoperatively (b-d) and their improvement postoperatively (e-g)
Figure 6.
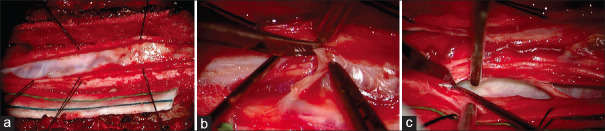
Intraoperative images from Case #2. Upon midline incision and lateral reflection of dural leaflets, a thick arachnoid web was noted covering the dorsal aspect of the spinal cord and restricting cerebrospinal fluid (CSF) flow (a). The thick arachnoid web was reflected caudad and resected using standard microsurgical techniques, revealing the normal vasculature of the spinal cord (b). The ventral arachnoid cyst on the left side was ruptured, restoring the dorsoventral and rostrocaudal CSF flow (c)
Discussion
AWs are rare, pathologic intradural extramedullary lesions of the spine that frequently present with weakness, sensory loss, urinary incontinence, or other myelopathic signs. Owing to the rarity of this entity, the diagnosis can present a particular challenge. Often, MRI findings lack the obvious signs of ACs, and “scalpel” sign, whereas the pia-arachnoid adhesions are not readily appreciated.[5] CT myelography can help identify myelographic blocks of CSF flow in the areas of the subdural space that otherwise look normal on MRI. Therefore, clinical correlation in patients with cord edema without obvious signs of ACs is critical. Thorough history and physical examinations with particular attention to past medical history are important in patients suspected of having AWs. While some studies suggested an association of AWs with a history of trauma or prior spine surgery, this relationship was observed only in 16% of cases in a systematic review.[3,4,5,6] SAH is being increasingly recognized in the literature as a risk factor for AW development.[7,8,9] We present two such instances of patients with a history of SAH then representing with spinal AWs, necessitating surgical resection.
The pathophysiology of AWs, particularly those arising after SAH,[3] lacks a unified theory and may therefore not be mentioned in the standard literature as a typical complication of SAH.[2] Migration of and layering of subarachnoid blood into the spinal subarachnoid space likely take place in patients with intracranial SAH; yet, most of those patients do not develop spinal complications. One theory suggests that subarachnoid blood breakdown sets off an inflammatory reaction that causes fibrous adhesions to form.[9] However, this presents an atypical feature, as the inflammatory process is generally diffuse rather than focal.[10,11] Therefore, it is unclear as to whether this inflammatory reaction is the true cause of AW or whether the inflammatory process worsens preexisting webs. Nevertheless, these adhesions can thicken the arachnoid layer and block the normal flow of CSF, possibly leading to the development of an AC. Both these fibrous adhesions and AC can disrupt the flow of CSF, which, in turn, can lead to cord edema, causing myelopathy. The onset of AW symptoms varies, with some manifesting as early as 3 months after spinal laminectomy and others at 2 years following a traumatic event.[3,5] Despite previous theories on AC or AW development post-SAH, the limited number of cases in the literature prevents any definitive conclusions.
Conclusion
Post-SAH AW is an increasingly recognized entity; however, the small number of cases is not enough to draw substantial conclusions on the pathophysiology of the development of AWs as it relates to SAH. Clinicians should be cognizant of delayed-onset myelopathy and back pain as potential signs of AW in patients with a history of SAH. By paying attention to these symptoms following an SAH, clinicians may identify additional individuals with AW, expanding the number of patients who could potentially benefit from therapeutic interventions.
Author contributions
GW: Literature search, data acquisition, data analysis, manuscript preparation, manuscript editing, manuscript review, guarantor; GP: Concepts, design, literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, manuscript review, guarantor; JB: Concepts, design, literature search, clinical studies, data acquisition, manuscript review, guarantor; ED: Concepts, design, literature search, clinical studies, manuscript review, guarantor; DF: Concepts, design, clinical studies, manuscript review, guarantor.
Ethical statement
Not applicable.
Declaration of Helsinki
In adherence to the Declaration of Helsinki, our research is conducted with the utmost respect for the dignity, safety, and rights of participants. We are committed to ensuring that our research protocols are ethically sound, scientifically justified, and transparent.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data availability statement
All data generated and/or analyzed during this study are included in this published article [and its supplementary information files].
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bosche B, Mergenthaler P, Doeppner TR, Hescheler J, Molcanyi M. Complex clearance mechanisms after intraventricular hemorrhage and rt-PA treatment-a review on clinical trials. Transl Stroke Res. 2020;11:337–44. doi: 10.1007/s12975-019-00735-6. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Papavassiliou E. Spinal intradural arachnoid webs causing spinal cord compression with inconclusive preoperative imaging: A report of 3 cases and a review of the literature. World Neurosurg. 2017;99:251–8. doi: 10.1016/j.wneu.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Nisson PL, Hussain I, Härtl R, Kim S, Baaj AA. Arachnoid web of the spine: A systematic literature review. J Neurosurg Spine. 2019;31:175–84. doi: 10.3171/2019.1.SPINE181371. [DOI] [PubMed] [Google Scholar]
- 5.Reardon MA, Raghavan P, Carpenter Bailey K, Mukherjee S, Smith JS, Matsumoto JA, et al. Dorsal thoracic arachnoid web and the “scalpel sign”: A distinct clinical-radiologic entity. AJNR Am J Neuroradiol. 2013;34:1104–10. doi: 10.3174/ajnr.A3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramore CG. Dorsal arachnoid web with spinal cord compression: Variant of an arachnoid cyst?Report of two cases. J Neurosurg. 2000;93:287–90. doi: 10.3171/spi.2000.93.2.0287. [DOI] [PubMed] [Google Scholar]
- 7.Brodbelt AR, Stoodley MA. Syringomyelia and the arachnoid web. Acta Neurochir (Wien) 2003;145:707–11. doi: 10.1007/s00701-003-0071-9. [DOI] [PubMed] [Google Scholar]
- 8.Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233–40. doi: 10.3171/jns.1997.86.2.0233. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez ML, Rodríguez EE, Millán JM, Urzaiz LL, Berrocal VR. Compressive myelopathy secondary to posthemorragic arachnoiditis: Case report and literature review. Clin Neurol Neurosurg. 2020;196:105964. doi: 10.1016/j.clineuro.2020.105964. [DOI] [PubMed] [Google Scholar]
- 10.Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, et al. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical study. Acta Neurochir (Wien) 1995;136:88–91. doi: 10.1007/BF01411441. [DOI] [PubMed] [Google Scholar]
- 11.Hammes EM., Jr Reaction of the meninges to blood. Arch Neurol Psychiatry. 1944;52:505–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article [and its supplementary information files].