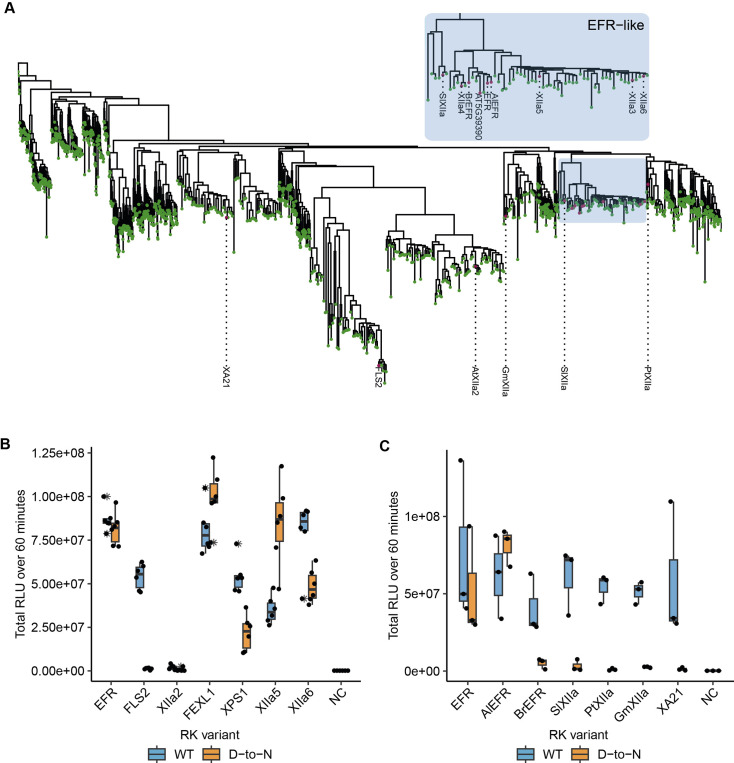
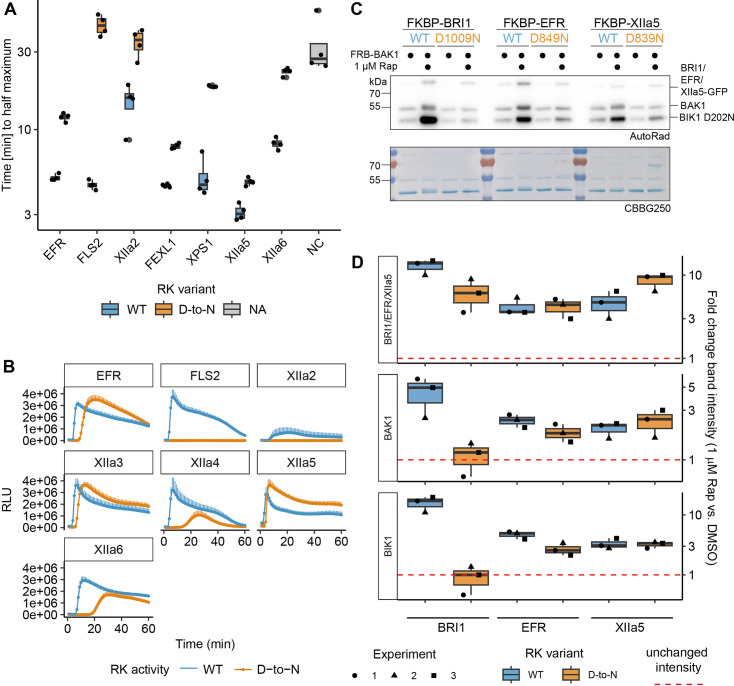
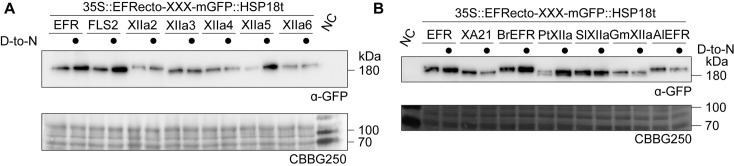
Figure 5. Related EFR kinases from LRR-RK XIIa in the Arabidopsis genus can function independent of their calatytic activity.
(A) Phylogenetic analysis of LRR-RK subfamily XIIa. Selected LRR-RK XIIa kinase domains are labeled and highlighted with purple points. The EFR-like clade contains all Arabidopsis XIIa kinases except FLS2 and XIIa2 and also selected XIIa kinases from Arabidopsis lyrata and Brassica rapa. (B, C) The ectodomain of EFR was fused to the transmembrane and intracellular domain of selected LRR-RK XIIa members to create elf18-responsive chimeras for testing the immune signaling function and catalytic dependency of the related kinase domains. The chimeras were transiently expressed in N. benthamiana and tested in oxidative burst assays. All Arabidopsis LRR-RK XIIa members induced an oxidative burst except XIIa2, the closest FLS2 related kinase in the subfamily. Catalytic dependency of the kinase domains appears to vary from kinase to kinase, with catalytically dead versions of EFR, FEXL1 and XIIa5 inducing a WT-like oxidative burst and XPS1 and XIIa6 displaying a reduced oxidative burst. FLS2 kinase dead exhibited a diminished oxidative burst. Experiments were repeated three times with similar results.