Figure 3.
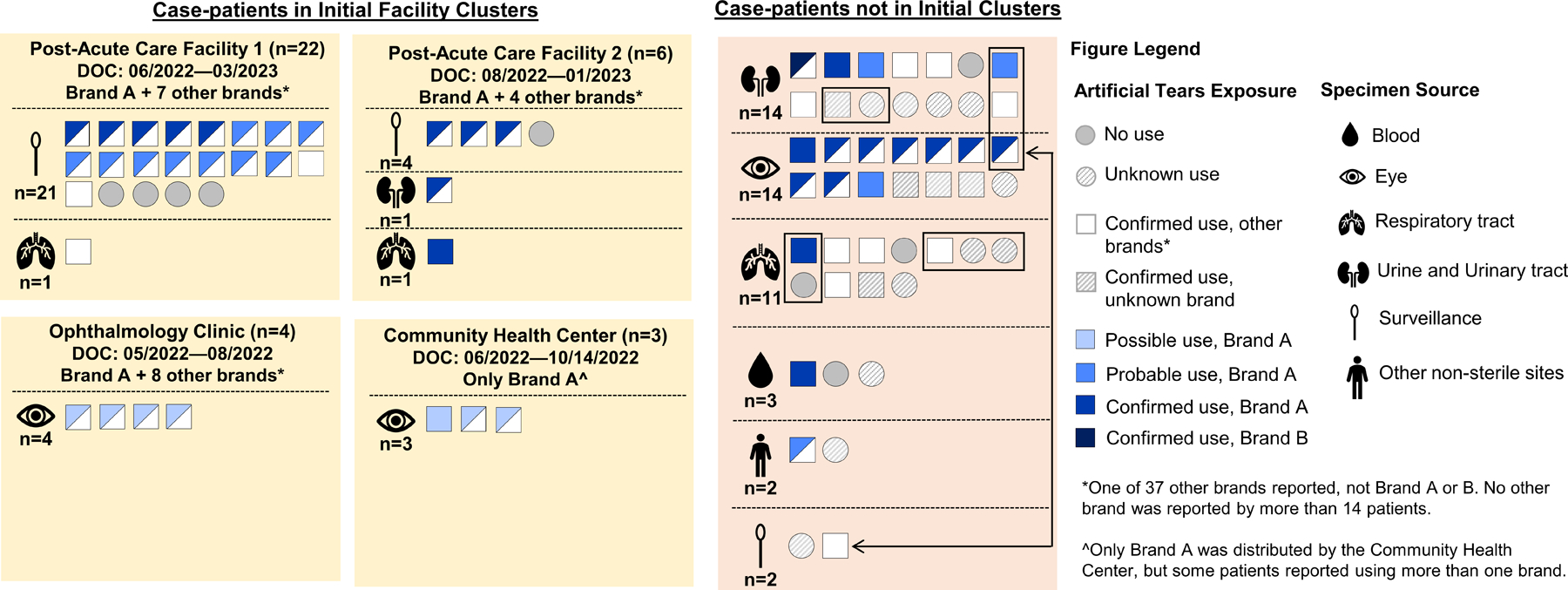
Artificial tears use among patients with P. aeruginosa ST1203 with blaVIM-80 and blaGES-9. Each square or circle represents an individual case-patient with use of artificial tears denoted by a shape and brand use indicated by color. Due to exposure uncertainty, we developed tiered definitions for brand exposure, which is shown only for Brands A and B; exposure to all other brands, regardless of exposure certainty, is shown in white. Exposure was confirmed if Brand A or B was documented in the medical record or reported by the case-patient or proxy. Exposure was probable if either (a) the case-patient received artificial tears in an inpatient facility that used Brand A or B concurrently with other brand(s) and did not document which brand(s) patients received, or (b) if a case-patient reported receiving a recall notice from a company or healthcare provider for artificial tears and could not remember the name of the recalled brand, but described a product compatible with Brand A or B. Exposure was possible if the case-patient received outpatient healthcare at a facility that was using Brand A or B during clinical care and/or providing Brand A or B to patients without documenting which brand(s) each patient received. Case-patients not in initial clusters who are epidemiologically linked are grouped with black rectangles or connected with a double-sided arrow.
