Abstract
Background & Aims
Sarcopenia is associated with increased morbidity and mortality in patients with cirrhosis, but its definition in current literature is very heterogeneous. We performed a systematic review and meta-analysis to assess the association between mortality and sarcopenia evaluated by computed tomography (CT) in patients with cirrhosis, both overall and stratified for the criteria used to define sarcopenia.
Methods
Medline, Embase, Scopus, and Cochrane Library were searched up to January 2023. We included studies assessing sarcopenia presence with CT scans and providing data on the risk of mortality. Adjusted hazard ratios (HRs) and 95% CIs were pooled using a random-effects model.
Results
Thirty-nine studies comprising 12,827 patients were included in the meta-analysis. The summary prevalence of sarcopenia was 44% (95% CI 38-50%). The presence of sarcopenia (any definition) was an independent predictor of mortality with an adjusted HR of 2.07 (95% CI 1.81-2.36), and the result was consistent in all subgroup analyses. The prognostic role of the EASL/AASLD criteria was confirmed for the first time with an HR of 1.86 (95% CI 1.53-2.26) (n = 14 studies). The cut-offs used to define sarcopenia based on psoas muscle parameters varied among studies, thus, a subgroup analysis was not feasible. There was no substantial heterogeneity for the main estimates and no significant risk of publication bias.
Conclusions
Sarcopenia on CT is associated with a 2-fold higher risk of mortality in patients with cirrhosis. The cut-offs proposed by EASL/AASLD are prognostically relevant and should be the recommended criteria used to define sarcopenia in clinical practice.
Impact and implications:
Sarcopenia assessed by the reference standard (computed tomography scan) is an independent predictor of mortality in patients with cirrhosis, with a 2-fold increase in the risk of death in all sensitivity analyses. This finding is particularly valid in patients from Europe and North America, and in transplant candidates. Stratifying for the parameters and cut-offs used, we confirmed for the first time the prognostic impact of the definition proposed by EASL/AASLD, supporting their use in clinical practice. Psoas muscle assessment is promising, but data are still limited and too heterogeneous to recommend its routine use at present.
Keywords: sarcopenia, malnutrition, skeletal muscle index, liver transplantation, survival, meta-analysis
Graphical abstract
Highlights:
-
•
A meta-analysis of 12,827 subjects found that sarcopenia was present in 44% (38-50%) of patients with cirrhosis.
-
•
Sarcopenia assessed by CT scan predicts overall survival, but its definition in the current literature is heterogenous.
-
•
For the first time, the prognostic relevance of the EASL/AASLD criteria was confirmed (HR 1.86, 95%-CI: 1.52-2.26, I2<25%).
-
•
Psoas muscle parameters may also be prognostically relevant, but the different cut-offs limit their use in clinical practice.
Introduction
Sarcopenia is defined as a progressive and generalized loss of muscle mass and function and is associated with increased morbidity and mortality.1 Cirrhosis is a major risk factor for the development of malnutrition and sarcopenia.2 In addition, the presence of sarcopenia in patients with cirrhosis increases the risk of infection, falls, decompensation, and mortality.[3], [4], [5] Over the past decade, efforts to identify patients at risk, refine the definition of sarcopenia in the specific context of liver disease, and propose interventions to improve muscle dysfunction have led to the first European and North American guidelines.6,7
Although the prognostic relevance of sarcopenia is undisputed,6 many questions remain unanswered from an operational and pragmatic point of view. First, the definition of sarcopenia in liver disease usually refers to the phenotypic manifestation of loss of muscle mass, but does not consider muscle quality or function.7 Second, the diagnosis of sarcopenia in the current literature is very heterogeneous in terms of tests (i.e. cross-sectional imaging, bioimpedance analysis), measures (i.e. psoas muscle thickness/index, skeletal muscle index [SMI], etc.), and cut-offs used, as clearly shown in a recent systematic review.8
Several meta-analyses have been published on this topic.[8], [9], [10], [11] However, many large and important studies have become available since the first meta-analysis by van Vugt et al. in 2016.9 In the most recent meta-analysis by Tantai et al.,8 the impact of sarcopenia on mortality was clearly demonstrated and was consistent in all subgroup analyses. However, the study did not stratify for the specific definition (test, cut-offs) of sarcopenia, making interpretation and application of the results in clinical practice challenging.
We performed a systematic review and meta-analysis to assess the prevalence of sarcopenia and the association between mortality and sarcopenia evaluated by computed tomography (CT), both overall and stratified for the criteria used to define sarcopenia, in patients with cirrhosis.
Material and methods
The meta-analysis was conducted and reported by the most recent MOOSE (Meta-analysis Of Observational Studies in Epidemiology) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) guidelines and was registered in PROSPERO (registration number: CRD42023387648).
Search strategy and selection criteria
We searched MEDLINE (via PubMed), Ovid Embase, Scopus, and the Cochrane Library databases up to 1st of January 2023. We used the following keywords: “sarcopenia”, “muscle wasting”, “skeletal muscle”, “cirrhosis”, and “liver disease”. The full search strategy is reported in Supplementary Material 1. Further searches were performed by manual review of references. The search and selection of studies were performed by two independent investigators (ED and FR), with a third (AC) arbitrating in case of conflict regarding the eligibility of a study for inclusion. A detailed full-text assessment of potentially relevant studies was then performed. Conference abstracts and letters to the editor were also included. There were no language restrictions.
Studies were included in the review if they met the following criteria: i) sarcopenia assessed by CT scan in patients with cirrhosis; ii) available data on mortality risk according to CT measures of sarcopenia. We excluded studies if they did not provide the essential information or included patients: i) with hepatocellular carcinoma (HCC) only; ii) undergoing TIPS (transjugular intrahepatic portosystemic shunt) placement; or iii) who were candidates for liver transplantation (LT) and the outcome was post-LT mortality. The most recent or most complete publication was considered when multiple articles were found for a single study to avoid duplication. However, some studies with overlapping cohorts were allowed in subgroup analyses, if they provided prognostic information for a specific definition of sarcopenia not included in the main study.
Data extraction and risk of bias assessment
A standardized extraction form was used to extract data from each included study. Two authors (ED and FR) extracted the following predefined data (if available): year of publication, study design, study location, number of patients, patient demographics, number of patients with viral etiology, patients with HCC, patients with decompensated cirrhosis, model for end-stage liver disease (MELD) score, main inclusion criteria (chronic liver disease, cirrhosis, acute-on-chronic liver failure [ACLF], LT candidates), definition of sarcopenia and association with mortality. If relevant data were not readily available, the authors were contacted to obtain additional data or clarification. Two authors (ED and FR) independently assessed the risk of bias using the Newcastle-Ottawa scale (NOS) for observational studies.
Sarcopenia definition
Muscle mass was quantified as a continuous variable either as SMI (cm2/m2) at the 3rd or 4th lumbar vertebra (L3/L4), or as a psoas muscle (PM)-based parameter (any of transversal psoas muscle thickness [TPMT, mm] measured at the umbilical level or L3, psoas muscle area [PMA, cm2] or index [PMI cm2/m2]).
The main definitions of sarcopenia evaluated were:
-
-
SMI <50 cm2/m2 in men and <39 cm2/m2 in women, as developed by Carey et al.12 in a cohort of LT candidates and endorsed by EASL and AASLD;6,7
-
-
SMI <52.4 cm2/m2 in men and <38.5 cm2/m2 in women, as proposed by Prado et al.13 in a cohort of patients with solid tumors of the respiratory and gastrointestinal tract;
-
-
SMI <53 cm2/m2 (or <43 cm2/m2 if body mass index <25 kg/m2) in men and <41 cm2/m2, as proposed by the same group as above in an updated paper by Martin et al.;14
-
-
SMI <42 cm2/m2 in men and <38 cm2/m2 in women, as proposed by the Japanese Society of Hepatology (JSH).15
Statistical analysis
The primary outcome was the association between the presence of sarcopenia (any definition) and mortality. Secondary outcomes were the association of sarcopenia (according to specific definitions and as a continuous value at CT measurement) with mortality.
The effect of sarcopenia on mortality was estimated by pooling in adjusted hazard ratios (HR) with 95% CIs using a random-effects model; if not available, unadjusted estimates were used.8,9 The variables included in the multivariate analyses of each study are summarized in Table S1. The prevalence of sarcopenia was pooled as binomial proportions with 95% CIs after Freeman-Tukey double arcsine transformation; differences between groups were tested using the random-effects meta-regression method. Heterogeneity between studies was assessed using the Q test and the I2 statistic; we considered an I2 value >50% as substantial heterogeneity.
Subgroup analyses were planned to examine the following sources of heterogeneity: region (Europe, North America, Latin America, Asia, Africa, Oceania), study design (retrospective vs. prospective), sample size (<150 vs. ≥150 patients), main etiology (viral vs. other), main inclusion criteria (cirrhosis vs. LT candidates vs. ACLF), inclusion of patients with HCC (yes vs. no), liver function (mean MELD <15 vs. ≥15), definition of sarcopenia (SMI- vs. PM-based), study quality. We also performed sensitivity analyses in which studies were pooled separately using competitive risk analysis (and reporting subhazard ratios [sHRs]). Publication bias was assessed using the funnel plot and Egger’s and Begg’s tests.
All analyses were performed with STATA version 17 (StataCorp, College Station, TX, USA) and R-Project version 4.1.1 (package meta and metafor, R Core Team 2021, Vienna, Austria).
Results
The electronic search identified 3,917 records after removing duplicates, of which 135 were assessed for eligibility. Of these, 33 were non-original studies, 34 assessed non-target populations (i.e., patients with HCC, at TIPS placement, or post-LT), 17 had insufficient data, and 12 had overlapping cohorts. Finally, 39 studies met the inclusion criteria[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54] and were included in the main analysis, representing a total of 12,827 patients. Data from eight additional studies12,[55], [56], [57], [58], [59], [60], [61] from the overlapping cohorts were used in the subgroup analyses. Fig. 1 shows the flowchart of the selection process and details the reasons for excluding studies. Almost all studies (37/39) were rated as high quality (NOS ≥7 points) (Table 1), so no sensitivity analysis based on study quality was performed.
Fig. 1.
PRISMA flowchart for included studies.
HCC, hepatocellular carcinoma; TIPS, transjugular portosystemic shunt.
Table 1.
Characteristics of included studies.
| Author, Year | Country | Study type | Patients | Main etiology | HCC, n (%) | Main inclusion criteria | Child-Pugh A, n (%) | MELD score | Main sarcopenia definition | Main cut-off (M, F)∗∗ | Cases, n (%) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anand et al., 2022 | India | Prospective, single center | 219 | ArLD | 0 | Cirrhosis, mixed | 108 (49%) | 12 | L3-SMI | <50, <39 | 170 (78%) | 8 |
| Benmassaoud et al., 2022 | UK | Retrospective, single center | 628 | Viral | 176 (28%) | LT candidates | 41 (7%) | 14 | L3-SMI | <50, <39 | 177 (28%) | 9 |
| Chen et al., 2022 | China | Retrospective, single center | 223 | Viral | 0 | Cirrhosis, mixed | 79 (35%) | 12 | L3-SMI | Continuous | N/A | 9 |
| Dajti et al., 2022 | Italy | Retrospective, single center | 209 | Viral | 40 (19%) | Cirrhosis, mixed | 175 (84%) | 10 | L3-SMI | <50, <39 | 134 (64%) | 7 |
| Khan et al., 2022 | India | Prospective, single center | 111 | ArLD | 0 | ACLF∗ | 0 (0%) | 28 | L3-SMI | <50, <39 | 76 (68%) | 6 |
| Kim et al., 2022 | Korea | Prospective, multicenter | 595 | Viral | 0 | Cirrhosis, mixed | 531 (89%) | 8 | L3-SMI | <50, <39 | 109 (18%) | 8 |
| Matsui et al., 2022 | Japan | Retrospective, single center | 202 | ArLD | 0 | Cirrhosis, mixed | N/A | 10 | L3-SMI | <42, <38 | 143 (71%) | 8 |
| Nardelli et al., 2022 | Italy | Prospective, single center | 114 | Viral | 0 | Cirrhosis, mixed | 32 (28%) | 13 | L3-SMI | <50, <39 | 68 (60%) | 8 |
| Peng et al., 2022 | China | Retrospective, single center | 433 | Viral | 0 | ACLF | N/A | 22 | L3-SMI | <42, <38 | 250 (58%) | 7 |
| Zeng et al., 2022 | China | Retrospective, multicenter | 480 | Viral | 0 | Cirrhosis, mixed | 112 (23%) | 12 | L3-SMI | <44.8, <32.5 | 109 (23%) | 7 |
| Cho et al., 2021 | Korea | Retrospective, single center | 166 | ArLD | 0 | Cirrhosis, mixed | 62 (37%) | 11 | L3-SMI | <50, <39 | 79 (48%) | 8 |
| Ishizu et al., 2021 | Japan | Retrospective, single center | 335 | Viral | 0 | Cirrhosis, mixed | 190 (57%) | N/A | L3-SMI | <42, <38 | 108 (32%) | 8 |
| Lai et al., 2021 | Italy | Retrospective, multicenter | 855 | Viral | 424 (50%) | LT candidates | N/A | 15 | L3-SMI | Continuous | N/A | 9 |
| Li et al., 2021 | China | Retrospective, single center | 171 | Viral | 0 | ACLF | N/A | 22 | L3-SMI | <42, <38 | 95 (56%) | 6 |
| Ruiz-Margain et al., 2021 | Mexico, USA | Retrospective, multicenter | 136 | Viral | 15 (11%) | Cirrhosis, mixed | 46 (34%) | 14 | L3-SMI | <50, <39 | 78 (57%) | 8 |
| Sidhu et al., 2021 | India | Prospective, single center | 161 | Viral | 0 | LT candidates | N/A | 22 | L3-SMI | Continuous | N/A | 8 |
| Hou et al., 2020 | China | Retrospective, single center | 274 | Viral | 0 | Cirrhosis, mixed | 102 (37%) | 12 | L3-SMI | <47, <32.5 | 100 (36%) | 9 |
| Kappus et al., 2020 | USA | Retrospective, single center | 355 | Viral | 95 (27%) | LT candidates | N/A | 19 | L3-SMI | <50, <39 | 61 (17%) | 8 |
| Kremer et al., 2020 | Germany | Prospective, single center | 87 | ArLD | 0 | Cirrhosis, mixed | 31 (36%) | 13 | L3-PMAr | <666, <424 | 44 (51%) | 8 |
| Mauro et al., 2020 | Argentina | Retrospective, single center | 180 | ArLD | 21 (12%) | LT candidates | 26 (14%) | 15 | L3-SMI | <50, <39 | 60 (33%) | 8 |
| Paternostro et al., 2020 | Austria | Retrospective, single center | 203 | ArLD | 0 | Cirrhosis, mixed | N/A | 12 | L3-TPMT | <12, <8 | 77 (38%) | 8 |
| Wang et al., 2020 | USA | Retrospective, single center | 254 | MASLD | 0 | Cirrhosis, mixed | 121 (48%) | 13 | L4-SMI | Continuous | N/A | 8 |
| Hamaguchi et al., 2019 | Japan | Retrospective, single center | 173 | Viral | 13 (8%) | LT candidates | N/A | 15 | L3-SMI | <40, <31 | 40 (23%) | 8 |
| Hanai et al., 2019 | Japan | Retrospective, single center | 563 | Viral | 397 (71%) | Cirrhosis, mixed | 375 (67%) | N/A | L3-SMI | <42, <38 | 118 (21%) | 8 |
| Lattanzi et al., 2019 | Italy | Retrospective, single center | 249 | Viral | 112 (45%) | Cirrhosis, mixed | N/A | 14 | L3-SMI | <50, <39 | 109 (44%) | 7 |
| Rodrigues et al., 2019 | Switzerland | Retrospective, single center | 84 | ArLD | 0 | Cirrhosis, mixed | N/A | 13 | L3-SMI | <50, <39 | 50 (60%) | 7 |
| Bhanji et al., 2018 | Canada | Retrospective, single center | 675 | Viral | 290 (43%) | LT candidates | 105 (16%) | 14 | L3-SMI | <50, <39 | 242 (36%) | 8 |
| Ebadi et al., 2018 | USA, Canada | Retrospective, multicenter | 353 | Mixed | 111 (31%) | LT candidates | N/A | 16 | L3-SMI | <50, <39 | 165 (47%) | 8 |
| Engelmann et al., 2018 | Germany | Retrospective, single center | 795 | ArLD | 173 (22%) | LT candidates | 112 (14%) | 16 | L3-SMI | Continuous | N/A | 9 |
| Gu et al., 2018 | Korea | Retrospective, multicenter | 653 | ArLD | 0 | Cirrhosis, mixed | N/A | 11 | L3-SMI | <52.4, <38.5 | 241 (37%) | 7 |
| Hiraoka et al., 2018 | Japan | Retrospective, single center | 346 | Viral | 118 (34%) | Cirrhosis, mixed | 230 (66%) | N/A | L3-PMI | <4.23, <2.5 | 54 (16%) | 7 |
| Huguet et al., 2018 | France | Retrospective, single center | 173 | ArLD | 0 | LT candidates | 17 (10%) | 21 | U-TPMT | <15.2 | 57 (33%) | 8 |
| Kang et al., 2018 | Korea | Retrospective, single center | 452 | ArLD | 0 | LT candidates | 215 (48%) | 9 | L3-SMI | <52.4, <38.5 | 190 (42%) | 8 |
| van Vugt et al., 2018 | Netherlands | Prospective, multicenter | 585 | Mixed | 193 (33%) | LT candidates | N/A | 14 | L3-SMI | <53/<43, <41 | 254 (43%) | 9 |
| Nishikawa et al., 2017 | Japan | Retrospective, single center | 206 | Viral | 53 (26%) | Cirrhosis, mixed | 140 (68%) | N/A | L3-PMI | <6.36, <3.92 | 117 (57%) | 8 |
| Sinclair et al., 2016 | Australia | Retrospective, single center | 145 | Viral | 33 (23%) | LT candidates | 10 (7%) | 18 | L4-SMI | Continuous | 102 (70%) | 9 |
| Wang et al., 2016 | USA | Prospective, single center | 292 | Viral | 134 (46%) | LT candidates | 79 (27%) | 15 | L3-SMI | Continuous | 111 (38%) | 9 |
| Hanai et al., 2015 | Japan | Retrospective, single center | 130 | Viral | 0 | Cirrhosis, mixed | 34 (26%) | N/A | L3-SMI | <52.4, <38.5 | 89 (68%) | 7 |
| Durand et al., 2014 | France | Retrospective, single center | 562 | Viral | 261 (46%) | LT candidates | N/A | 14 | U-TPMT | Continuous | N/A | 8 |
ACLF, acute-on-chronic liver failure; ArLD, alcohol-related liver disease; LT, liver transplant; MASLD, metabolic dysfunction-associated steatosis liver disease; N/A, not available; NOS, Newcastle-Ottawa scale; PMAr, psoas muscle area; PMI, psoas muscle index; SMI, skeletal muscle index; TPMT, transversal psoas muscle thickness.
Critically ill cirrhotic patients in the intensive care unit.
Unit for SMI and PMI: cm2/m2, TPMT: mm.
Characteristics of the included studies
The characteristics of the included studies are shown in Table 1. Briefly, 19 studies were conducted in Asia,16,18,[20], [21], [22],[24], [25], [26], [27],29,31,32,38,39,45,46,48,50,53 12 studies in Europe,17,19,23,28,34,36,40,41,44,47,49,54 six studies in North America,30,33,37,42,43,52 one in Latin America (Argentina)35 and one in Oceania (Australia).51 Only eight studies were prospective,16,20,21,23,31,34,37,49 and the rest were retrospective. The mean age of patients included varied from 4316 to 71 years39 and the proportion of male patients ranged from 40%30 to 100%.51 The proportion of patients with viral etiology ranged from 9%49 to 72%;46 21 studies did not include patients with HCC and in the remaining studies the prevalence of HCC ranged from 8%38 to 71%.39 The main inclusion criterion was cirrhosis (both compensated and decompensated) in 21 studies, LT candidates in 15 studies, and ACLF in three studies.
Prevalence of sarcopenia
The summary prevalence of sarcopenia was 44% (95% CI 38-50%, I2 = 97.2%) (Fig. S1, Table S2). In the subgroup analyses, the prevalence of sarcopenia increased progressively with increasing disease severity, according to the main inclusion criteria: 37% (95% CI 30-44%, I2 = 95.2%) in studies including compensated and decompensated patients with cirrhosis, 46% (95% CI 37-55%, I2 = 97.7%) in LT candidates and 60% (95% CI 53-66%, I2 not evaluable) in patients with ACLF. The summary prevalence was also lower in large studies (≥150 patients) (39%, 95% CI 33-45%, I2 = 59.6%) than in smaller series (<150 patients) (63%, 95% CI 57-68%, I2 = 97.7%). No other significant differences were found in the other subgroup and meta-regression analyses (Table S2).
Effect of sarcopenia on mortality
From the 30 studies (n = 9,404 patients) that provided data, the presence of sarcopenia was independently associated with a 2-fold increased risk of mortality, with a summary adjusted HR of 2.07 (95% CI 1.81-2.36, I2 = 34.2%) (Table 2, Fig. S2). This association was robust and remained significant with a similar HR (range 1.9-2.4) in all prespecified subgroup analyses (i.e. study size and design, study location, main etiology, inclusion of patients with HCC, severity of liver disease, definition of sarcopenia), except in the small subgroup analysis (n = 3 studies) of patients with ACLF (summary HR 2.31, 95% CI 0.92-5.76, I2 = 82.4%) (Table 2).
Table 2.
Association between sarcopenia and mortality.
| Subgroup | Sarcopenia presence (dichotomized variable) |
Skeletal muscle index (continuous variable) |
||||
|---|---|---|---|---|---|---|
| Studies, n | Summary HR (95% CI) | I2 | Studies, n | Summary HR (95% CI) | I2 | |
| Overall summary | 30 | 2.07 (1.81-2.36) | 34.2% | 20 | 0.98 (0.97-0.98) | 37.5% |
| Study type | ||||||
| Retrospective | 24 | 2.07 (1.76-2.34) | 42.4% | 4 | 0.98 (0.97-0.99) | 34.5% |
| Prospective | 6 | 2.04 (1.52-2.73) | 0% | 16 | 0.94 (0.90-0.97) | 0% |
| Study size | ||||||
| <150 patients | 5 | 2.441 (1.66-3.59) | 0% | 3 | 0.97 (0.95-0.99) | 0% |
| >150 patients | 25 | 2.03 (1.76-2.35) | 34.2% | 17 | 0.98 (0.97-0.99) | 43.1% |
| Region | ||||||
| Asia | 17 | 2.16 (1.76-2.64) | 49.6% | 7 | 0.97 (0.94-0.99) | 57.8% |
| Europe | 9 | 1.81 (1.48-2.22) | 0% | 5 | 0.99 (0.98-1) | 0% |
| North America | 3 | 2.01 (1.46-2.77) | 16% | 7 | 0.97 (0.96-0.98) | 20.3% |
| Main etiology | ||||||
| Viral | 18 | 2.11 (1.73-2.57) | 51.8% | 14 | 0.98 (0.97-0.99) | 45.6% |
| ArLD | 11 | 2.09 (1.70-2.57) | 0% | 4 | 0.97 (0.95-0.99) | 20.5% |
| Inclusion of HCC patients | ||||||
| No | 18 | 2.10 (1.74-2.53) | 31.7% | 8 | 0.97 (0.95-0.99) | 52.8% |
| Yes | 12 | 2.03 (1.66-2.48) | 41% | 12 | 0.98 (0.97-0.99) | 29.2% |
| Main inclusion criteria | ||||||
| Cirrhosis | 17 | 2.09 (1.75-2.49) | 29.4% | 11 | 0.98 (0.97-0.99) | 42% |
| LT candidates | 11 | 1.99 (1.66-2.28) | 9.7% | 8 | 0.97 (0.96-0.98) | 0% |
| ACLF | 3 | 2.31 (0.92-5.76) | 82.4% | N/A | N/A | N/A |
| Liver function | ||||||
| Mean MELD <15 | 17 | 2.01 (1.76-2.29) | 0% | 10 | 0.98 (0.97-0.99) | 11.3% |
| Mean MELD ≥15 | 8 | 2.30 (1.55-3.41) | 58.1% | 9 | 0.97 (0.96-0.99) | 60.5% |
| Definition used | ||||||
| SMI-based | 24 | 2.00 (1.73-2.31) | 30% | N/A | N/A | N/A |
| PM-based | 6 | 2.43 (1.68-3.51) | 52.8% | N/A | N/A | N/A |
ACLF, acute-on-chronic liver failure; ArLD, alcohol-related liver disease; CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; LT, liver transplant; MELD, model for end-stage liver disease; N/A, not available; PM, psoas muscle; SMI, skeletal muscle index.
Impact of sarcopenia defined by SMI on mortality
Twenty studies (n = 7,314 patients) provided data to estimate the risk of death using SMI as a continuous variable. Each 1 cm2/m2 increase in SMI was significantly associated with a reduced risk of death, with a summary adjusted HR of 0.98 (95% CI 0.97-0.98, I2 = 37.5%) (Fig. S3). This association remained significant in all the prespecified subgroup analyses (Table 2).
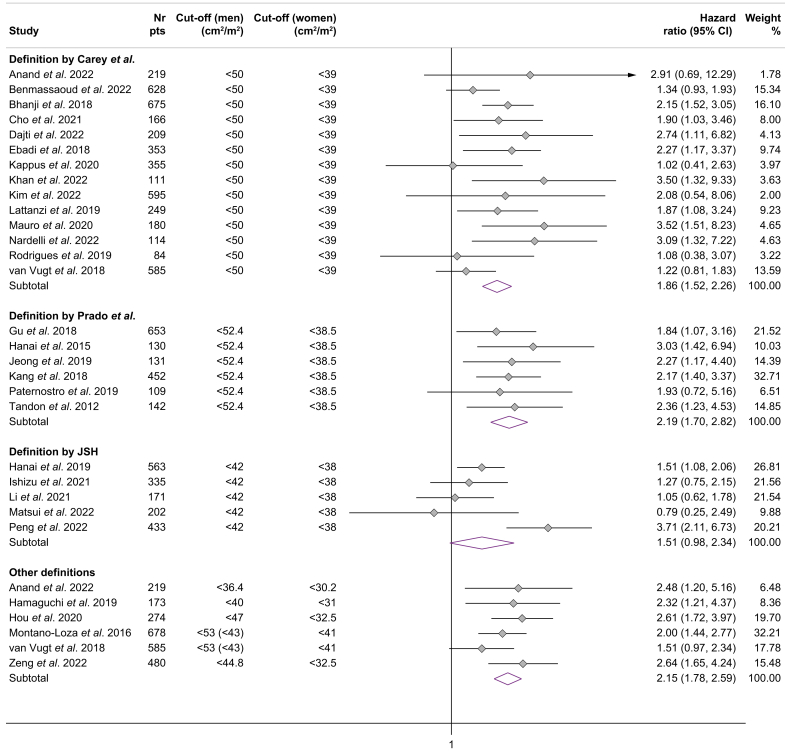
The association between mortality and the presence of sarcopenia (dichotomized) according to the most commonly used cut-offs is summarized in Fig. 2. The presence of sarcopenia defined by the EASL/AASLD criteria (Carey et al.12) increased the risk of mortality with a summary adjusted HR of 1.86 (95% CI 1.52-2.26) without substantial heterogeneity (I2 = 24.3%) (n = 14 studies). Similarly, sarcopenia as defined by Prado et al.13 (n = 6 studies) was independently associated with increased mortality (summary adjusted HR 2.19, 95% CI 1.70-2.82, I2 = 0%). In contrast, the association between the JSH criteria and mortality was not significant in a subgroup analysis of five studies (summary adjusted HR 1.51, 95% CI 0.98-2.34, I2 = 68.4%). The subgroup analysis for the Martin et al.14 definition of sarcopenia was not possible due to the limited number of studies (n = 2) providing data.
Fig. 2.
Association between mortality and sarcopenia defined according to published skeletal muscle cut-offs.
Impact of sarcopenia defined by psoas muscle-based parameters on mortality
Higher values of both PMI (cm2/m2) and TPMT (mm) (continuous variables) were associated with a decreased risk of mortality, with summary adjusted HRs of 0.88 (95% CI 0.77-0.99, I2 = 81.4%) and 0.92 (95% CI 0.86-0.98, I2 = 0%), respectively (Table 3).
Table 3.
Association between mortality and psoas muscle-based variables.
| Variable | Studies, n | Summary HR (95% CI) | I2 |
|---|---|---|---|
| PMI (continuous variable) | 5 | 0.88 (0.77-0.99) | 81.4% |
| TPMT (continuous variable) | 4 | 0.92 (0.86-0.98) | 0% |
| Psoas muscle parameter (any) (continuous variable) | 10∗ | 0.88 (0.83-0.95) | 80.8% |
| PMI (dichotomous variable) | 6 | 2.32 (1.69-3.19) | 55.2% |
| TPMT (dichotomous variable) | 4 | 2.50 (1.48-4.23) | 52.5% |
| Sarcopenia (any definition) (dichotomous variable) | 11∗ | 2.29 (1.81-2.90) | 43% |
HR, hazard ratio; PMI, psoas muscle index; TPMT, transversal psoas muscle thickness.
One study evaluated psoas muscle area.
Similarly, the presence of sarcopenia (dichotomous variable) defined by these parameters was significantly associated with an increased risk of mortality (Fig. 3). However, subgroup analysis per paired cut-offs was not possible as each study used different cut-offs to define sarcopenia.
Fig. 3.
Association between mortality and sarcopenia defined according to published cut-offs based on psoas muscle.
Sensitivity analysis and risk of bias
As HRs from Cox-proportional hazard regression may overestimate the risk of death, we planned a sensitivity analysis by separately pooling HRs and sHRs (competing-risk analysis). This analysis was only possible for the presence of sarcopenia (any definition) and sarcopenia according to the EASL/AASLD criteria, with summary adjusted sHRs of 2.05 (95% CI 1.64-2.57, I2 = 0%) and 2.07 (95% CI 1.47-2.93, I2 = 43.8%), respectively. The minimum number of studies for subgroup analysis was not reached for the other definitions of sarcopenia.
The funnel plot for the primary outcomes was symmetrical (Fig. S4). Egger’s and Begg’s tests were respectively 0.259 and 0.239, suggesting that there was no potential publication bias.
Discussion
To our knowledge, this is the first study to systematically review and synthesize the prognostic relevance of sarcopenia according to available measures and definitions of sarcopenia on CT scans. In a meta-analysis including 39 studies and almost 13,000 patients with cirrhosis, we demonstrated that sarcopenia is an independent predictor of mortality, with an adjusted 2.1-fold increase in the risk of death. These findings were robust in all subgroup analyses, with no substantial heterogeneity. All CT-based measures of sarcopenia were independent predictors of mortality: the risk of death increased by 2.4%, 8%, and 12.4% per unit decrease in L3-SMI, TPMT, and PMI, respectively. More importantly, after stratification according to published sarcopenia cut-offs, we confirmed for the first time the prognostic relevance of the EASL/AASLD definition of sarcopenia (L3-SMI <50 cm2/m2 in men and <39 cm2/m2 in women). This approach was not possible for psoas muscle-based definitions, as all included studies used different cut-offs to define sarcopenia, so these parameters cannot currently be recommended for use in clinical practice.
A strength of this systematic review is the comprehensive search of the literature without restrictions on language, type of publication, and number of patients included. We carefully selected among studies with overlapping cohorts, pooled adjusted estimates, and performed multiple subgroup analyses to confirm the robustness of our results. The main strengths of our analysis are the inclusion of only CT-based criteria to evaluate sarcopenia, reducing the heterogeneity between studies, and stratification for the most commonly used paired cut-offs to define sarcopenia. In addition, the test for publication bias was not statistically significant, minimizing the risk of unpublished studies influencing our results.
A weakness of our findings is the heterogeneity between studies, especially for the estimates of sarcopenia prevalence (I2 >75%). However, the heterogeneity was not substantial (I2 <50%) for the main outcome and was low (I2 <25%) for prospective studies, studies conducted in Europe and North America, studies including LT candidates, and for the definition of sarcopenia according to EASL/AASLD criteria. Of note, the number and type of confounding factors included in multivariate analyses varied significantly among studies. This could explain in part the heterogeneity among studies, and it cannot be analyzed unless individual patient data are used.
Finally, most of the included studies were retrospective and single center, and are therefore subject to selection bias; however, the overall quality was rated as high in 95% of the studies.
To our knowledge, this is the first meta-analysis to separately analyze the different CT definitions of sarcopenia and to separately evaluate the EASL/AASLD criteria. The first meta-analysis by van Vugt et al.9 was published in 2015 and, similar to our study, only evaluated sarcopenia by CT scan. However, only six studies were included in this meta-analysis (four with overlapping cohorts) and many papers have become available since its publication. Other meta-analyses10,11 were limited by the inclusion of overlapping cohorts, the inclusion of post-LT patients, heterogeneous modalities for assessing sarcopenia, and high heterogeneity for the main estimates. The most recent and important meta-analysis on this topic was published by Tantai et al.8; it included only high-quality studies with at least 100 patients and showed an independent association between sarcopenia and (waiting list) mortality with an adjusted HR of 2.30 (95% CI 2.01-2.63, n = 16 studies), with no heterogeneity between studies (I2 = 0). However, the assessment of sarcopenia in this meta-analysis was very heterogeneous, both in terms of modality (cross-sectional imaging, bioimpedance analysis, dual-energy X-ray absorptiometry) and definitions (SMI- vs. PM-based cut-offs). These differences were not taken into account in their analyses, thus limiting the interpretation of the results. In this respect, the stratified analysis of parameters and paired cut-offs for the diagnosis of sarcopenia is the main novelty of our meta-analysis. Other differences between the two papers include: i) inclusion of studies assessing sarcopenia only by CT scan in our study, the gold standard for assessing sarcopenia in hepatology; ii) the exclusion of studies with <100 patients and a significant publication bias found in the paper by Tantai et al.; iii) exclusion of studies with an empirical proportion of patients with HCC >50%8; and iv) total number of patients included (12,827 patients from 39 studies vs. 6,965 patients from 22 studies, of whom 5,840 were evaluated with CT scan).
Sarcopenia is a common complication in patients with cirrhosis and an independent predictor of mortality. These results are particularly valid in Europe and North America and in LT candidates. More importantly, the subgroup analysis for the different definitions of sarcopenia confirmed for the first time the cut-offs proposed by Carey et al.12 and endorsed by EASL/AASLD6,7; the summary adjusted HR was 1.86 (95% CI 1.52-2.26) with low heterogeneity between studies. Similar results were found using the definition of sarcopenia by Prado et al.13 However, given the similarity between the cut-offs used in these two definitions (<50 vs. <52.4 cm2/m2 in men and <39 vs. <38 cm2/m2 in women), it could be argued that the EASL/AASLD criteria, derived specifically in a large cohort of patients with cirrhosis, should be the reference standard to be used in clinical practice. The JSH criteria15 showed a borderline association with mortality risk in our subgroup analysis, but these results should be interpreted with caution. In fact, sarcopenia is defined in Asian countries as a loss of both muscle mass and muscle function, and many studies were excluded from the analysis because they did not provide data for muscle mass separately. Finally, the assessment of the psoas muscle could be a promising and more rapid tool to assess sarcopenia, as it does not require a dedicated software to calculate. However, the cut-offs used in the current literature were different, which prevented the conduction of subgroup analyses and the recommendation of their use in routine clinical practice.
In conclusion, our data confirm that sarcopenia on CT scan is an independent predictor of mortality in patients with cirrhosis. We confirmed for the first time the prognostic relevance of the EASL/AASLD criteria for sarcopenia, but not that of the psoas muscle-based definitions, due to the different cut-offs used in the published studies; thus, the former may be the preferred criteria to assess sarcopenia in cirrhosis. Future well-designed, high-quality studies are needed to validate these results in real life and in specific contexts (e.g. patients with compensated cirrhosis, patients with ACLF) and geographical areas.
Abbreviations
ACLF, acute-on-chronic liver failure; CI, confidence interval; CT, computed tomography; HCC, hepatocellular carcinoma; HR, hazard ratio; JSH, Japanese Society of Hepatology; LT, liver transplantation; MELD, model for end-stage liver disease; PMI, psoas muscle index; SMI, skeletal muscle index; TPMT, transversal psoas muscle index.
Financial support
No grants or other financial support.
Conflict of interest
The authors of this study declare that they do not have any conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
ED, FR, AB, AC formulated the research questions and developed the study protocol. ED, SGR, FP, LC, GM, FR collected and extracted the data, with AB, AC, FA, GB, MR and ED providing supervision and guarantors of the study. ED, SGR, and FR analysed the data. ED, SGR, FP, LC, GM, MR, GB, FA, AB, AC, FR wrote the manuscript. All authors had full access to all the data in the study, reviewed the manuscript, and had final responsibility for the decision to submit for publication.
Data availability statement
Data available upon reasonable request from the authors.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101113.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis european working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandon P., Montano-Loza A.J., Lai J.C., et al. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75:S147–S162. doi: 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapper E.B., Nikirk S., Parikh N.D., et al. Falls are common, morbid, and predictable in patients with cirrhosis. J Hepatol. 2021;75(3):582. doi: 10.1016/j.jhep.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marasco G., Dajti E., Ravaioli F., et al. Clinical impact of sarcopenia assessment in patients with liver cirrhosis. Expert Rev Gastroenterol Hepatol. 2021;15(4):377–388. doi: 10.1080/17474124.2021.1848542. [DOI] [PubMed] [Google Scholar]
- 5.Ge J., Kim W.R., Lai J.C., et al. Beyond MELD” - emerging strategies and technologies for improving mortality prediction, organ allocation and outcomes in liver transplantation. J Hepatol. 2022;76(6):1318–1329. doi: 10.1016/j.jhep.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merli M., Berzigotti A., Zelber-Sagi S., et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai J.C., Tandon P., Bernal W., et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74(3):1611–1644. doi: 10.1002/hep.32049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tantai X., Liu Y., Yeo Y.H., et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76(3):588–599. doi: 10.1016/j.jhep.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 9.van Vugt J.L.A., Levolger S., de Bruin R.W.F., et al. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transpl. 2016;16(8):2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 10.Kim G., Kang S.H., Kim M.Y., et al. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang K.V., Chen J De, Wu W.T., et al. Association of loss of muscle mass with mortality in liver cirrhosis without or before liver transplantation: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98(9) doi: 10.1097/MD.0000000000014373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey E.J., Lai J.C., Wang C.W., et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23(5):625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prado C.M., Lieffers J.R., McCargar L.J., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 14.Martin L., Birdsell L., MacDonald N., et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa H., Shiraki M., Hiramatsu A., et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46(10):951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 16.Anand A., Mohta S., Agarwal S., et al. European working group on sarcopenia in older people (EWGSOP2) criteria with population-based skeletal muscle index best predicts mortality in asians with cirrhosis. J Clin Exp Hepatol. 2022;12(1):52–60. doi: 10.1016/j.jceh.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benmassaoud A., Roccarina D., Arico F.M., et al. Sex is a major effect modifier between body composition and mortality in patients with cirrhosis assessed for liver transplantation. Liver Int. 2023;43(1):160–169. doi: 10.1111/liv.15293. [DOI] [PubMed] [Google Scholar]
- 18.Chen C., Chu Y., Sheng Q., et al. Skeletal muscle index and muscle attenuation with liver cirrhosis as survival prognosticators by sex. Asia Pac J Clin Nutr. 2022;31(1):24–32. doi: 10.6133/apjcn.202203_31(1).0003. [DOI] [PubMed] [Google Scholar]
- 19.Dajti E., Renzulli M., Ravaioli F., et al. The interplay between sarcopenia and portal hypertension predicts ascites and mortality in cirrhosis: sarcopenia predicts ascites and death in cirrhotic patients. Dig Liver Dis. 2023;55(5):637–643. doi: 10.1016/j.dld.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Khan S., Benjamin J., Maiwall R., et al. Sarcopenia is the independent predictor of mortality in critically ill patients with cirrhosis. J Clin Transl Res. 2022;8(3):200. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim T.H., Jung Y.K., Yim H.J., et al. Impacts of muscle mass dynamics on prognosis of outpatients with cirrhosis. Clin Mol Hepatol. 2022;28(4):876–889. doi: 10.3350/cmh.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui T., Nagai H., Watanabe G., et al. Measurement of skeletal muscle volume is useful for predicting prognosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2022;34(11):1151–1157. doi: 10.1097/MEG.0000000000002435. [DOI] [PubMed] [Google Scholar]
- 23.Nardelli S., Riggio O., Gioia S., et al. Risk factors for hepatic encephalopathy and mortality in cirrhosis: the role of cognitive impairment, muscle alterations and shunts: predictors of HE. Dig Liver Dis. 2022;54(8):1060–1065. doi: 10.1016/j.dld.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Peng H., Zhang Q., Luo L., et al. A prognostic model of acute-on-chronic liver failure based on sarcopenia. Hepatol Int. 2022;16(4):964–972. doi: 10.1007/s12072-022-10363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng X., Shi Z.W., Yu J.J., et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12(6):1948–1958. doi: 10.1002/jcsm.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho Y.S., Lee H.Y., Jeong J.Y., et al. Computed tomography-determined body composition abnormalities usefully predict long-term mortality in patients with liver cirrhosis. J Comput Assist Tomogr. 2021;45(5):684–690. doi: 10.1097/RCT.0000000000001207. [DOI] [PubMed] [Google Scholar]
- 27.Ishizu Y., Ishigami M., Honda T., et al. Impact of visceral fat accumulation on the prognosis of patients with cirrhosis. Clin Nutr ESPEN. 2021;42:354–360. doi: 10.1016/j.clnesp.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Lai Q., Magistri P., Lionetti R., et al. Sarco-Model: a score to predict the dropout risk in the perspective of organ allocation in patients awaiting liver transplantation. Liver Int. 2021;41(7):1629–1640. doi: 10.1111/liv.14889. [DOI] [PubMed] [Google Scholar]
- 29.Li T., Xu M., Kong M., et al. Use of skeletal muscle index as a predictor of short-term mortality in patients with acute-on-chronic liver failure. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-92087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Margáin A., Xie J.J., Román-Calleja B.M., et al. Phase angle from bioelectrical impedance for the assessment of sarcopenia in cirrhosis with or without ascites. Clin Gastroenterol Hepatol. 2021;19(9):1941–1949.e2. doi: 10.1016/j.cgh.2020.08.066. [DOI] [PubMed] [Google Scholar]
- 31.Sidhu S.S., Saggar K., Goyal O., et al. Muscle strength and physical performance, rather than muscle mass, correlate with mortality in end-stage liver disease. Eur J Gastroenterol Hepatol. 2021;33(4):555–564. doi: 10.1097/MEG.0000000000001761. [DOI] [PubMed] [Google Scholar]
- 32.Hou L., Deng Y., Fan X., et al. A sex-stratified prognostic nomogram incorporating body compositions for long-term mortality in cirrhosis. JPEN J Parenter Enteral Nutr. 2021;45(2):403–413. doi: 10.1002/jpen.1841. [DOI] [PubMed] [Google Scholar]
- 33.Kappus M.R., Wegermann K., Bozdogan E., et al. Use of skeletal muscle index as a predictor of wait-list mortality in patients with end-stage liver disease. Liver Transpl. 2020;26(9):1090–1099. doi: 10.1002/lt.25802. [DOI] [PubMed] [Google Scholar]
- 34.Kremer W.M., Nagel M., Reuter M., et al. Validation of the clinical frailty scale for the prediction of mortality in patients with liver cirrhosis. Clin Transl Gastroenterol. 2020;11(7) doi: 10.14309/ctg.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauro E., Crespo G., Martinez-Garmendia A., et al. Cystatin C and sarcopenia predict acute on chronic liver failure development and mortality in patients on the liver transplant waiting list. Transplantation. 2020;11(7) doi: 10.1097/TP.0000000000003222. [DOI] [PubMed] [Google Scholar]
- 36.Paternostro R., Bardach C., Hofer B.S., et al. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int. 2021;41(4):799–809. doi: 10.1111/liv.14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N.C., Zhang P., Tapper E.B., et al. Automated measurements of muscle mass using deep learning can predict clinical outcomes in patients with liver disease. Am J Gastroenterol. 2020;115(8):1210–1216. doi: 10.14309/ajg.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamaguchi Y., Kaido T., Okumura S., et al. Including body composition in MELD scores improves mortality prediction among patients awaiting liver transplantation. Clin Nutr. 2020;39(6):1885–1892. doi: 10.1016/j.clnu.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Hanai T., Shiraki M., Imai K., et al. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: a sex-stratified analysis. Hepatol Res. 2019;49(12):1414–1426. doi: 10.1111/hepr.13420. [DOI] [PubMed] [Google Scholar]
- 40.Lattanzi B., Nardelli S., Pigliacelli A., et al. The additive value of sarcopenia, myosteatosis and hepatic encephalopathy in the predictivity of model for end-stage liver disease. Dig Liver Dis. 2019;51(11):1508–1512. doi: 10.1016/j.dld.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues S.G., Brabandt B., Stirnimann G., et al. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int. 2019;39(9):1672–1681. doi: 10.1111/liv.14175. [DOI] [PubMed] [Google Scholar]
- 42.Bhanji R.A., Moctezuma-Velazquez C., Duarte-Rojo A., et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12(4):377–386. doi: 10.1007/s12072-018-9875-9. [DOI] [PubMed] [Google Scholar]
- 43.Ebadi M., Wang C.W., Lai J.C., et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle. 2018;9(6):1053–1062. doi: 10.1002/jcsm.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelmann C., Schob S., Nonnenmacher I., et al. Loss of paraspinal muscle mass is a gender-specific consequence of cirrhosis that predicts complications and death. Aliment Pharmacol Ther. 2018;48(11–12):1271–1281. doi: 10.1111/apt.15026. [DOI] [PubMed] [Google Scholar]
- 45.Gu D.H., Kim M.Y., Seo Y.S., et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24(3):319–330. doi: 10.3350/cmh.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiraoka A., Kitahata S., Izumoto H., et al. Muscle volume loss a prognostic factor for death in liver cirrhosis patients and special relationship to portal hypertension. Hepatol Res. 2018;48(3):E354–E359. doi: 10.1111/hepr.12984. [DOI] [PubMed] [Google Scholar]
- 47.Huguet A., Latournerie M., Debry P.H., et al. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: a retrospective cohort study. Nutrition. 2018;51–52:73–79. doi: 10.1016/j.nut.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Kang S.H., Jeong W.K., Baik S.K., et al. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. 2018;9(5):860–870. doi: 10.1002/jcsm.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Vugt J.L.A., Alferink L.J.M., Buettner S., et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018;68(4):707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 50.Nishikawa H., Enomoto H., Ishii A., et al. Prognostic significance of low skeletal muscle mass compared with protein-energy malnutrition in liver cirrhosis. Hepatol Res. 2017;47(10):1042–1052. doi: 10.1111/hepr.12843. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair M., Grossmann M., Angus P.W., et al. Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol. 2016;31(3):661–667. doi: 10.1111/jgh.13182. [DOI] [PubMed] [Google Scholar]
- 52.Wang C.W., Feng S., Covinsky K.E., et al. A comparison of muscle function, mass, and quality in liver transplant candidates: results from the functional assessment in liver transplantation study. Transplantation. 2016;100(8):1692–1698. doi: 10.1097/TP.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanai T., Shiraki M., Nishimura K., et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31(1):193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Durand F., Buyse S., Francoz C., et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60(6):1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Hou L., Deng Y., Wu H., et al. Low psoas muscle index associates with long-term mortality in cirrhosis: construction of a nomogram. Ann Transl Med. 2020;8(6):358. doi: 10.21037/atm.2020.02.49. 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paternostro R., Lampichler K., Bardach C., et al. The value of different CT-based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019;39(12):2374–2385. doi: 10.1111/liv.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong J.Y., Lim S., Sohn J.H., et al. Presence of sarcopenia and its rate of change are independently associated with long-term mortality in patients with liver cirrhosis. J Korean Med Sci. 2018;33(50) doi: 10.3346/jkms.2018.33.e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishizu Y., Ishigami M., Kuzuya T., et al. Low skeletal muscle mass predicts early mortality in cirrhotic patients with acute variceal bleeding. Nutrition. 2017;42:87–91. doi: 10.1016/j.nut.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Montano-Loza A.J., Angulo P., Meza-Junco J., et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim T.Y., Kim M.Y., Sohn J.H., et al. Sarcopenia as a useful predictor for long-term mortality in cirrhotic patients with ascites. J Korean Med Sci. 2014;29(9):1253–1259. doi: 10.3346/jkms.2014.29.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tandon P., Ney M., Irwin I., et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18(10):1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request from the authors.