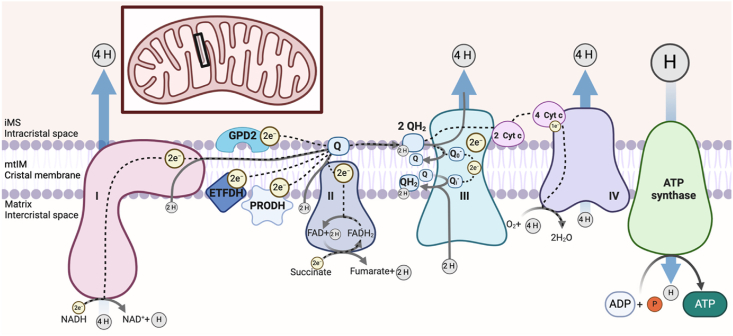
Figure 2.
Overview of the electron transfer system and pathways of oxidative phosphorylation.
The cristal membrane of the mitochondrial inner membrane (mtIM) is the primary site of oxidation and reduction reactions within both the electron transfer system (ETS) and oxidative phosphorylation (OXPHOS). The respiratory sequence initiates with oxidation of 3 NADH molecules at Complex I and succinate at Complex II. Of note, depending on the substrate, complete oxidation can yield more than 1 full turn of the tricarboxylic acid (TCA) cycle. Depending on the anaplerotic/cataplerotic status of cycle intermediates, a substrate may not undergo complete oxidation and thus a partial TCA cycle may occur. NADH oxidation to NAD+ +H+ transfers 2 electrons (e−) per NADH through the large Complex resulting in the transfer of 4 protons (H+) from the matrix into the intermembrane space (iMS) through Complex I. In contrast, Complex II oxidizes succinate to fumarate, transferring 2e− to bound FAD, yielding FADH2, which subsequently transfers the electrons to the Q-junction without hydrogen ion pumping. The electrons are passed from Complexes I and II to Q, an electron carrier, which is reduced to QH2, utilizing protons from the matrix. Electrons are transferred through the nonpolar region of the phospholipid mtIM bilayer to Complex III and protons are released into the iMS. Other membrane-bound structures contribute to net electron transfer in a tissue and organism-specific manner, including glycerol-3-phosphate dehydrogenase (GPD2), the electron transferring flavoprotein dehydrogenase (ETFDH), and proline dehydrogenase (PRODH). The complete transfer of 2 e− from Q through Complex III to cytochrome c requires 2 QH2 molecules and 2 cytochrome c molecules in a two-step process. 4 H+ from both QH2 molecules are pumped into the IMS and 2 H+ from the matrix are used to reform 1 QH2, resulting in a net proton gradient change of 6 H+. Each cytochrome c then transports an electron to Complex IV through the sequential reduction of a copper and heme group, requiring a total of 4 reduced cytochrome c molecules and allowing an oxygen molecule to bind and receive a total of 4e−. Additionally, four H+ from the matrix are pumped through Complex IV into the IMS and four H+ from the matrix are used to form two H2O, resulting in a net proton gradient change of eight H+. The established electrochemical proton gradient between the matrix and the IMS pushes protons through ATP synthase, down the gradient, back into the matrix. With the proton movement, ATP synthase spins and phosphorylates ADP with inorganic phosphate (Pi) to make ATP. However, with increased concentrations of ATP or if respiration is compromised and the pmF falls, the ATP synthase reaction can reverse and hydrolyze ATP to pump protons back out of the matrix to re-establish the pmF. Ultimately the efficiency of the OXPHOS system is influenced by electrochemical gradients, substrate and oxygen concentration, and the integrity of the respiratory Complexes.