Abstract
Introduction
The JAVELIN Lung 101 phase 1b/2 trial evaluated avelumab (immune checkpoint inhibitor) combined with lorlatinib or crizotinib (tyrosine kinase inhibitors) in ALK-positive or ALK-negative advanced NSCLC, respectively.
Methods
Starting doses of lorlatinib 100 mg once daily or crizotinib 250 mg twice daily were administered with avelumab 10 mg/kg every 2 weeks. Primary objectives were assessment of maximum tolerated dose (MTD) and recommended phase 2 dose in phase 1 and objective response rate in phase 2. Primary end points were dose-limiting toxicity (DLT) and confirmed objective response per Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
In the avelumab plus lorlatinib group (ALK-positive; n = 31; 28 in phase 1b; three in phase 2), two of 28 assessable patients (7%) had DLT, and the MTD and recommended phase 2 dose was avelumab 10 mg/kg every 2 weeks plus lorlatinib 100 mg once daily. In the avelumab plus crizotinib group (ALK-negative; n = 12; all phase 1b), five of 12 assessable patients (42%) had DLT, and the MTD was exceeded with avelumab 10 mg/kg every 2 weeks plus crizotinib 250 mg twice daily; alternative crizotinib doses were not assessed. Objective response rate was 52% (95% confidence interval, 33%–70%) with avelumab plus lorlatinib (complete response, 3%; partial response, 48%) and 25% (95% confidence interval, 6%–57%) with avelumab plus crizotinib (all partial responses).
Conclusions
Avelumab plus lorlatinib treatment in ALK-positive NSCLC was feasible, but avelumab plus crizotinib treatment in ALK-negative NSCLC could not be administered at the doses tested. No evidence of increased antitumor activity was observed in either group.
ClinicalTrials.gov identifier
Keywords: Non–small cell lung cancer, Avelumab, Lorlatinib, Crizotinib, Phase 1b/2
Introduction
Treatment strategies for patients with advanced NSCLC are based on consideration of targetable driver-gene alterations, programmed death-ligand 1 (PD-L1) expression, and tumor histology.1,2 Tyrosine kinase inhibitors (TKIs) are an established treatment option for patients whose tumors have actionable gene alterations, with ALK TKIs used for tumors with ALK rearrangements (ALK positive). For patients whose tumors do not harbor actionable gene alterations, guidelines recommend several immune checkpoint inhibitor (ICI)-based regimens, including ICI monotherapy for patients whose tumors have high PD-L1 expression and ICI–based combinations for other patients.1,2
Crizotinib is a multitargeted ALK TKI that also suppresses c-MET and ROS1 and was the first drug approved for ALK-positive metastatic NSCLC; however, most patients receiving crizotinib develop disease progression due to ALK mutations, up-regulation of bypass signaling pathways, or central nervous system metastasis.3, 4, 5 Although second-generation ALK TKIs have been developed, including ceritinib, alectinib, and brigatinib, disease progression due to ALK mutations occurs in more than half of treated patients.4,6, 7, 8 Lorlatinib is an approved third-generation, central nervous system–penetrant ALK TKI with activity against ALK mutations that develop during treatment with crizotinib or other ALK TKIs.9, 10, 11
Avelumab is an anti–PD-L1 ICI approved in various countries as monotherapy for metastatic Merkel cell carcinoma, first-line maintenance treatment (in patients whose disease has not progressed with first-line platinum-containing chemotherapy) and second-line treatment for locally advanced or metastatic urothelial carcinoma, and first-line treatment for advanced renal cell carcinoma in combination with axitinib.12,13 In a phase 1b cohort of the JAVELIN Solid Tumor trial, avelumab was found to have antitumor activity and an acceptable safety profile as first-line treatment in patients with EGFR- or ALK-negative advanced NSCLC.14 In a phase 3 trial of avelumab versus docetaxel as second-line treatment for PD-L1–positive advanced NSCLC (JAVELIN Lung 200), overall survival (OS) differences did not reach statistical significance in the overall population, but 2-year OS rates were doubled with avelumab in subgroups defined by tumors with higher PD-L1 expression.15,16 In a phase 3 trial of avelumab versus platinum doublet chemotherapy as first-line treatment for PD-L1–high NSCLC (JAVELIN Lung 100), differences in OS and progression-free survival (PFS; primary end points) were not statistically significant.17
TKIs have various immunomodulatory effects in the tumor microenvironment,18 providing a rationale for assessing ICI plus TKI combinations in patients with ALK-positive or ALK-negative advanced NSCLC. For example, ALK rearrangements induce PD-L1 expression, raising the possibility that ICI plus ALK TKI combination treatment might have enhanced activity in ALK-positive NSCLC.19, 20, 21, 22, 23, 24 In addition, ALK inhibition has been found to have immunostimulatory effects, including increased CD8-positive T-cell accumulation in tumors in vivo and promotion of T-cell interactions with cancer cells ex vivo.25,26 In clinical studies, ICI plus ALK TKI combinations (nivolumab + crizotinib; nivolumab + ceritinib; atezolizumab + alectinib; pembrolizumab + crizotinib) were found to have an antitumor activity, although some combinations had increased toxicity compared with single agents27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38; however, studies of ICI plus lorlatinib treatment in patients with ALK-positive tumors are needed. Furthermore, interactions between c-MET and its ligand hepatocyte growth factor induce immunosuppressive effects in various immune cell types within the tumor microenvironment, which can be reversed by c-MET inhibition.39 Thus, inhibition of both ALK and c-MET by crizotinib provides a rationale for evaluating ICI plus crizotinib combination treatment in patients with ALK-negative NSCLC.
Here, we report results from the JAVELIN Lung 101 phase 1b/2 dose-finding trial in patients with locally advanced or metastatic NSCLC, which evaluated the safety, efficacy, and pharmacokinetics (PK) of avelumab in combination with lorlatinib in patients with ALK-positive tumors or in combination with crizotinib in patients with ALK-negative tumors.
Materials and Methods
Study Design and Patients
JAVELIN Lung 101 (NCT02584634) was a phase 1b/2, multicenter, open-label, dose-finding study. Eligible patients were aged at least 18 years (≥20 y in Japan) and had locally advanced or metastatic NSCLC; one or more measurable lesion per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) that had not previously been irradiated; Eastern Cooperative Oncology Group performance status of 0 to 2; life expectancy of at least 3 months; adequate bone marrow, renal, liver, and pancreatic functions (absolute neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L, hemoglobin ≥ 109 g/dL, estimated creatinine clearance ≥ 30 mL/min according to the Cockcroft-Gault formula, total bilirubin level ≤ 1.5× the upper limit of normal [ULN], aspartate aminotransferase [AST] or alanine aminotransferase [ALT] ≤ 2.5 × ULN, and serum amylase or lipase < 1.5 × ULN); no prior immunotherapy; and available tumor tissue (archived tissue or fresh biopsy).
In the phase 1b portion, patients in the avelumab plus lorlatinib group had ALK-positive tumors and any number of prior regimens (including zero); prior treatment with lorlatinib was permitted. In the phase 2 portion, the avelumab plus lorlatinib group was limited to patients with no prior systemic treatment for advanced or metastatic disease (adjuvant or neoadjuvant treatment was permitted if completed ≥6 mo before study entry) and no prior TKI treatment at any time. PD-L1 status was not considered in eligibility criteria. Patients in the avelumab plus crizotinib group were previously treated and had ALK-negative tumors with no ROS1 translocations or c-MET amplification or exon 14 skipping mutations; patients with EGFR mutations were permitted if they had disease progression after prior EGFR inhibitor treatment.
Exclusion criteria included major surgery within 4 weeks or radiation therapy within 2 weeks before study entry (prior palliative radiotherapy was permitted if completed ≥48 h before patient registration); systemic cytotoxic anticancer therapy within 2 weeks or TKI therapy within 5 half-lives before study entry; brain metastases with some exceptions (patients with brain metastases were eligible for the avelumab plus crizotinib group if they had completed treatment and recovered from acute effects of radiotherapy or surgery before study entry, had discontinued corticosteroid treatment for ≥4 wk, and were neurologically stable; patients with brain metastases were eligible for the avelumab plus lorlatinib group if they had asymptomatic metastases requiring <10 mg/d prednisone or equivalent); persistent toxicity greater than grade 1 related to previous therapy (excluding grade 2 alopecia); diagnosis of any other malignancy within 3 years before study entry (except for adequately treated basal or squamous cell skin cancer, carcinoma in situ of the breast or cervix, or low-grade prostate cancer [Gleason 6 or below] without any plans for treatment intervention); and prior use of immunosuppressive medication within 7 days before randomization (except for intranasal, inhaled, or topical steroids or local steroid injections; systemic corticosteroids at physiological doses; or steroids as premedication for hypersensitivity).
This trial was conducted in accordance with the ethical principles of the Declaration of Helsinki and the Good Clinical Practice guidelines defined by the International Council for Harmonisation. All participating patients provided written informed consent. The protocol was approved by the institutional review board or independent ethics committee at each participating center.
Procedures
Avelumab 10 mg/kg was administered every 2 weeks by intravenous (IV) infusion in both treatment groups. Lorlatinib was administered orally as 25 mg tablets, with a starting dose of 100 mg once daily. Crizotinib was administered orally as 250 mg capsules, with a starting dose of 250 mg twice daily. Patients were treated until disease progression, unacceptable toxicity, or other protocol-specified criteria for withdrawal occurred. Discontinuation of one drug due to treatment-related toxicity with continuation of treatment as monotherapy was permitted outside of the dose-limiting toxicity (DLT) period. Follow-up periods were 30, 60, and 90 days after the last study treatment.
End Points and Assessments
The primary objective in phase 1b was to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) for avelumab in combination with either lorlatinib or crizotinib. The primary objective in phase 2 was to assess the objective response rate (ORR) in all patients and the complete response (CR) rate in the avelumab plus lorlatinib group per RECIST 1.1. The primary end point in phase 1b was occurrence of DLT within the first 2 treatment cycles (14-d cycle). The primary end point in phase 2 was confirmed objective response per RECIST 1.1 by investigator assessment (including CR in the avelumab plus lorlatinib group). Secondary end points included safety, OS, PK, tumor tissue biomarkers, duration of response (DOR; assessed from CR or partial response [PR] until progressive disease [PD], death, or last tumor assessment), disease control rate (DCR), time to response (TTR), and PFS, all assessed by investigator per RECIST 1.1.
The MTD was the highest dose of avelumab plus lorlatinib or crizotinib associated with DLT in fewer than 33% of patients within the first 2 cycles of treatment. DLT was defined as the occurrence of any of the following adverse events (AEs) attributable to one or more drug during the primary DLT observation period: (1) hematologic AEs comprising grade 4 neutropenia lasting more than 7 days; febrile neutropenia; grade 3 or higher neutropenic infection; grade 3 or higher thrombocytopenia with bleeding; grade 4 thrombocytopenia; and grade 4 anemia; (2) nonhematologic AEs comprising any toxicity of grade 3 or higher except for the following: transient (≤6 h) grade 3 flu-like symptoms or fever controlled with medical management; transient (≤24 h) grade 3 fatigue, local reactions, or headache; grade 3 nausea or vomiting that resolved to grade 1 or lower within 7 days with medical management; grade 3 diarrhea or skin toxicity that resolved to grade 1 or lower within 7 days with medical management; grade 3 or higher amylase or lipase abnormality not associated with pancreatitis; tumor flare phenomenon (local pain, irritation, or rash localized at tumor site); or an abnormal laboratory value that is unlikely related to study treatment; and (3) inability to complete 75% of lorlatinib or crizotinib treatment or two infusions of avelumab due to treatment-related toxicities.
AEs and laboratory test abnormalities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 and coded using the Medical Dictionary for Regulatory Activities. Infusion-related reaction (IRR) was analyzed as an AE of special interest using a composite definition that included the following preferred terms: infusion-related reaction, chills, pyrexia, and dyspnea. Antitumor activity was assessed radiologically at screening, at week 8, and then every 8 weeks until PD. Tumor responses were confirmed at least 4 weeks after initial documentation of a CR or PR. PD-L1 status was determined centrally using the Ventana PD-L1 (SP263) immunohistochemistry assay (Roche Diagnostics, Indianapolis, IN); PD-L1–positive status was defined as a combined positive score (tumor and immune cells) of at least 1%.
PK parameters of avelumab, lorlatinib, and crizotinib were evaluated using blood samples. For PK analyses of avelumab, 3.5-mL samples were collected before infusion and 1 hour after infusion on day 1 and at any time on day 8 of cycles 1 and 2. Additional samples were collected on day 1 of cycles 3 to 5, followed by day 1 every 6 cycles thereafter, and at the end of treatment. For PK analyses of lorlatinib and crizotinib, samples (4 mL and 3 mL, respectively) were collected predose and at 1, 2, 4, 6, and 8 hours postdose on day 1 of cycle 2. Sparse blood samples were collected predose on day 1 of cycles 4 and 7. For all patients enrolled in phase 2, predose blood samples were collected on day 1 of cycles 2, 4, and 7.
Safety and efficacy were assessed in all patients who received at least one dose of the study drug (safety analysis set and full analysis set). DLT was evaluated in all patients in phase 1b who received at least one dose of the study drug and either had DLT during the first 2 cycles of treatment or completed the observation period for the first 2 cycles; patients without DLT who received less than 75% of the doses of lorlatinib or crizotinib in the first 2 cycles or less than two avelumab infusions for reasons other than study drug-related toxicity were replaced. Response according to PD-L1 status was assessed in the biomarker analysis set, which included all patients in the safety analysis set who had at least one baseline biomarker assessment and had received at least one dose of any study drug. Kaplan-Meier estimates were used for time-to-event analyses, and the confidence interval (CI) for the median was calculated according to Brookmeyer and Crowley methodology.
PK parameters were assessed in the PK analysis set, which included all patients in the safety analysis set who had sufficient concentration data to estimate at least one PK parameter of interest. Trough plasma concentration (Ctrough) and maximum plasma concentration (Cmax) for avelumab, lorlatinib, and crizotinib were summarized descriptively (n, mean, standard deviation, percentage of coefficient of variation [CV], median, minimum, maximum, geometric mean, CV, and 95% CI) by treatment group, cycle, and day.
Other PK parameters included time to maximum plasma concentration, time of last measurable concentration, area under the plasma concentration-time curve during the dosing interval time course, area under the concentration-time curve from time of dosing to the last collection time point, and apparent plasma clearance (CL/F). For the metabolite of crizotinib, metabolite-to-parent ratio for area under the plasma concentration versus time curve during the dosing interval time course and the metabolite-to-parent ratio for Cmax were also determined. Dose-normalized parameters for lorlatinib and crizotinib (including its metabolite) were also determined.
Results
Patient Characteristics and Disposition
Between December 18, 2015, and March 6, 2018, 66 patients were screened and 43 were enrolled at 16 centers across the United States, Australia, France, Spain, South Korea, and Japan. Patients received either avelumab plus lorlatinib (n = 31; 28 in phase 1b and three in phase 2) or avelumab plus crizotinib (n = 12; all in phase 1b). In both treatment groups, all patients received starting doses (no other dose levels were administered). Most patients were Asian, had Eastern Cooperative Oncology Group performance status of 1 or 2, had PD-L1–positive tumors, had tumors with nonsquamous histology, and had received at least two prior anticancer drug regimens (Table 1). Most patients (27 of 31) in the avelumab plus lorlatinib group had received prior therapy with an ALK TKI, and most patients (eight of 12) in the avelumab plus crizotinib group had a history of smoking.
Table 1.
Patient Baseline Characteristics
| Characteristic | Avelumab + Lorlatinib (n = 31) | Avelumab + Crizotinib (n = 12) |
|---|---|---|
| Age, median (range), y | 54 (30–77) | 60 (43–76) |
| Sex, n (%) | ||
| Female | 19 (61) | 6 (50) |
| Male | 12 (39) | 6 (50) |
| Race, n (%) | ||
| Asian | 17 (55) | 8 (67) |
| White | 13 (42) | 4 (33) |
| American Indian or Alaska Native | 1 (3) | 0 |
| ECOG PS, n (%) | ||
| 0 | 11 (36) | 3 (25) |
| 1 | 17 (55) | 9 (75) |
| 2 | 3 (10) | 0 |
| History of smoking, n (%) | ||
| Current | 0 | 1 (8) |
| Former | 6 (19) | 7 (58) |
| Never | 25 (81) | 4 (33) |
| PD-L1 status, n (%)a | ||
| Positive | 20 (65) | 7 (58) |
| Negative | 4 (13) | 2 (17) |
| Unknown | 7 (23) | 3 (25) |
| Histopathologic classification, n (%) | ||
| Adenocarcinoma | 28 (90) | 11 (92) |
| Squamous cell carcinoma | 1 (3) | 1 (8) |
| Other | 1 (3) | 0 |
| Prior anticancer drug regimens, n (%) | ||
| 0 | 4 (13) | 0 |
| 1 | 6 (19) | 2 (17) |
| ≥2 | 21 (68) | 10 (83) |
| Prior anticancer drug regimens for advanced or metastatic disease, n (%) | ||
| 0 | 5 (16) | 2 (17) |
| 1 | 7 (23) | 2 (17) |
| ≥2 | 19 (61) | 8 (67) |
| Prior ALK TKI regimens, n (%) | ||
| 0 | 4 (13) | 12 (100) |
| 1 | 9 (29) | 0 |
| ≥2 | 18 (58) | 0 |
ECOG PS, Eastern Cooperative Oncology Group performance status; IHC, immunohistochemistry; PD-L1, programmed death-ligand 1; TKI, tyrosine kinase inhibitor.
PD-L1–positive status was defined as a combined positive score of at least 1% (Ventana PD-L1 [SP263] IHC assay).
On July 13, 2022 (data cutoff date), the study was stopped, and five patients with ongoing avelumab plus lorlatinib treatment were moved to a continued access study (NCT05059522); no patient had ongoing avelumab plus crizotinib treatment. The most common reason for permanent treatment discontinuation in both treatment groups was PD (Supplementary Table 1). Median follow-up for OS was 42 months in the avelumab plus lorlatinib group and 35 months in the avelumab plus crizotinib group. Median duration of exposure in the avelumab plus lorlatinib group was 42 weeks (range, 2–308 wk) for avelumab and 46 weeks (range, 2–308 wk) for lorlatinib; in the avelumab plus crizotinib group, median duration of exposure was 15 weeks (range, 4–126 wk) for avelumab and 6 weeks (range, 2–32 wk) for crizotinib. In the avelumab plus lorlatinib and avelumab plus crizotinib groups, 13 of 31 (42%) and seven of 12 (58%) patients received subsequent anticancer treatment, respectively.
DLT and Safety
In DLT-assessable patients in the avelumab plus lorlatinib group (n = 28), two patients (7%) had a DLT (ALT increased in both patients; Table 2). The MTD and RP2D was determined to be avelumab 10 mg/kg IV every 2 weeks plus lorlatinib 100 mg once daily, and administration of avelumab in combination with lorlatinib at these doses was deemed feasible.
Table 2.
DLT in Assessable Patients
| Parameter, (n%) | Avelumab + Lorlatinib (n = 28) | Avelumab + Crizotinib (n = 12) |
|---|---|---|
| DLT | 2 (7) | 5 (42) |
| ALT increased | 2 (7) | 2 (17) |
| AST increased | 0 | 2 (17) |
| ECG QT prolonged | 0 | 1 (8) |
| Febrile neutropenia | 0 | 1 (8) |
| Immune-mediated hepatitis | 0 | 1 (8) |
| Rash | 0 | 1 (8) |
Note: Patients with more than one DLT are counted only once.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLT, dose-limiting toxicity; ECG, electrocardiogram.
In patients assessable for safety in the avelumab plus lorlatinib group (n = 31), treatment-emergent AEs (TEAEs) occurred in 30 patients (97%), including grade 3 or higher TEAEs in 23 patients (74%) (Table 3). The most common any-grade TEAE was blood cholesterol increased in 19 patients (61%) (Supplementary Table 2). IRR occurred in nine patients (29%). The most common grade 3 or higher TEAEs were lipase increased and hypertriglyceridemia in four patients (13%) each. Treatment-related AEs (TRAEs) occurred in 28 patients (90%), including grade 3 or higher TRAEs in 16 patients (52%). Serious TEAEs occurred in 21 patients (68%), and the most common serious TEAE (≥2 patients) was pneumonia in three patients (10%) (Supplementary Table 3). TEAEs leading to death occurred in four patients (13%), including sudden death, NSCLC, cerebral hemorrhage, and dyspnea (n = 1 each); only dyspnea was considered related to the treatment (Table 3).
Table 3.
Safety Summary
| AE, n (%) | Avelumab + Lorlatinib (n = 31) | Avelumab + Crizotinib (n = 12) |
|---|---|---|
| TEAE, any grade | 30 (97) | 12 (100) |
| Grade ≥3 | 23 (74) | 7 (58) |
| TRAE, any grade | 28 (90) | 12 (100) |
| Grade ≥3 | 16 (52) | 6 (50) |
| TEAE leading to permanent discontinuation of any study drug | 10 (32) | 6 (50) |
| TEAE leading to permanent discontinuation of all study drugs | 1 (3) | 3 (25) |
| TRAE leading to permanent discontinuation of avelumab | 9 (29) | 2 (17) |
| TRAE leading to permanent discontinuation of crizotinib | 0 | 5 (42) |
| TRAE leading to permanent discontinuation of lorlatinib | 2 (7) | 0 |
| TEAE leading to death | 4 (13) | 1 (8) |
| TRAE leading to death | 1 (3) | 0 |
| IRR (AE of special interest) | 9 (29) | 5 (42) |
AE, adverse event; IRR, infusion-related reaction; TEAE, treatment-emergent AE; TRAE, treatment-related AE.
In DLT-assessable patients in the avelumab plus crizotinib group, five of 12 patients (42%) had DLT, and the most common AEs resulting in DLT were ALT increased and AST increased (two of 12 patients [17%] each) (Table 2). The MTD was exceeded with avelumab 10 mg/kg IV every 2 weeks plus crizotinib 250 mg twice daily. Because of the high occurrence of DLTs in this group, it was decided that alternative doses would not be assessed and that the MTD could not be identified; thus, the prespecified criterion to proceed to phase 2 (observation of treatment responses at the MTD) was not met.
In patients assessable for safety in the avelumab plus crizotinib group (n = 12), TEAEs occurred in all patients, including grade 3 or higher TEAEs in seven patients (58%) (Table 3). The most common any-grade TEAE was nausea in seven patients (58%), which was considered treatment related in all patients (Supplementary Table 2). IRR (AE of special interest) occurred in five patients (42%). The most common grade 3 or higher TEAE was ALT increased in two patients (17%), which was considered treatment related in both patients. TRAEs occurred in all patients, including grade 3 or higher TRAEs in six patients (50%). Serious TEAEs occurred in five patients (42%) in the avelumab plus crizotinib group, with no single serious TEAE occurring in more than one patient (Supplementary Table 3). One patient (8%) had a TEAE that led to death (disease progression, considered unrelated to treatment; Table 3).
Efficacy
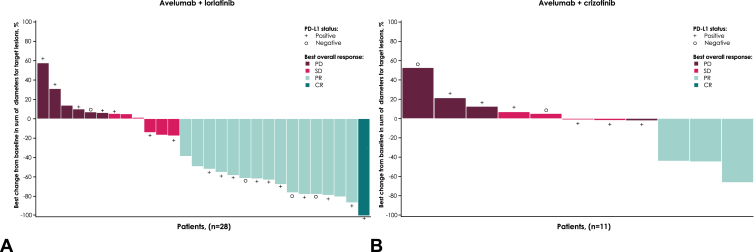
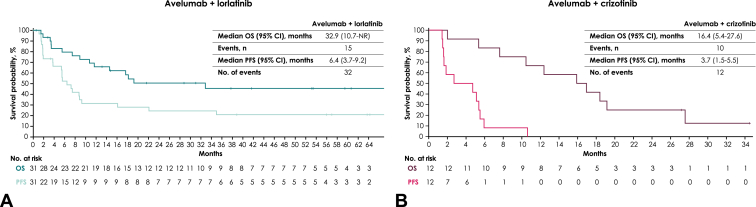
In the avelumab plus lorlatinib group (n = 31), the confirmed ORR was 51.6% (95% CI, 33%–70%); one patient (3%) had a CR and 15 patients (48%) had a PR (Table 4). Median TTR was 1.8 months (range, 1.3–3.7 mo), and median DOR was 14.7 months (range, 3.7 mo–not assessable). The DCR with avelumab plus lorlatinib was 71% (95% CI, 52%–86%). Best percentage change from baseline in target lesions is shown in Figure 1A. Median PFS and median OS were 6.4 months (95% CI, 3.7–9.2 mo) and 32.9 months (95% CI, 10.7 mo–not assessable), respectively (Fig. 2A). The 2-year OS rate was 50% (95% CI, 31%–67%).
Table 4.
Summary of Responses per Investigator Assessment
| Response | Avelumab + Lorlatinib (n = 31) | Avelumab + Crizotinib (n = 12) |
|---|---|---|
| Confirmed best overall response, n (%) | ||
| CR | 1 (3) | 0 |
| PR | 15 (48) | 3 (25) |
| SD | 6 (19) | 4 (33) |
| PD | 7 (23) | 5 (42) |
| NE | 2 (7) | 0 |
| ORR (95% CI), % | 52 (33–70) | 25 (6–57) |
| Median TTR (range), mo | 1.8 (1.3–3.7) | 1.4 (1.4–6.9) |
| Median DOR (95% CI), mo | 14.7 (3.7–NE) | 3.7 (3.7–4.6) |
| DCR (95% CI), % | 71 (52–86) | 58 (28–85) |
CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; TTR, time to response.
Figure 1.
Best percentage change from baseline in sum of diameters for target lesions per investigator assessment in the (A) avelumab plus lorlatinib and (B) avelumab plus crizotinib groups. CR, complete response; PD, progressive disease; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease.
Figure 2.
Kaplan-Meier analysis of OS and PFS in the (A) avelumab plus lorlatinib and (B) avelumab plus crizotinib groups. OS, overall survival; NR, not reached; PFS, progression-free survival.
In the avelumab plus crizotinib group, the confirmed ORR was 25% (95% CI, 6%–57%) based on PR in three of 12 patients (no CRs; Table 4). Median TTR was 1.4 months (range, 1.4–6.9 mo), and median DOR was 3.7 months (range, 3.7–4.6 mo). The DCR with avelumab plus crizotinib was 58% (95% CI, 28%–85%). Best percentage change from baseline in target lesions is shown in Figure 1B. Median PFS and median OS were 3.7 months (95% CI, 1.5–5.5 mo) and 16.4 months (95% CI, 5.4–27.6 mo), respectively (Fig. 2B). The 2-year OS rate was 25% (95% CI, 6%–50%).
Biomarker Analyses
In the avelumab plus lorlatinib group, 24 patients were assessable for PD-L1 status; in the PD-L1–positive (n = 20) and PD-L1–negative (n = 4) subgroups, the ORR was 50% and 75% and the DCR was 65% and 75%, respectively (Supplementary Table 4). In the avelumab plus crizotinib group, nine patients were assessable for PD-L1 status; none of the patients had a response, and the DCR in the PD-L1–positive (n = 7) and PD-L1–negative (n = 2) subgroups was 43% (95% CI, 0%–41%) and 50% (95% CI, 1%–99%), respectively.
PK Analyses
In patients who received avelumab in combination with lorlatinib or crizotinib, the Cmax for avelumab was similar on cycle 1 day 1 and cycle 2 day 1, and Ctrough level reached steady state above 10 μg/mL at cycle 2 (Supplementary Fig. 1). The steady-state plasma PK of lorlatinib in combination with avelumab was similar to that of previously reported exposure with lorlatinib monotherapy (Supplementary Table 5).10 Serum concentrations of avelumab were similar across patients in either treatment group. Exposures for crizotinib and its metabolite in combination with avelumab were similar to those reported for crizotinib monotherapy (Supplementary Tables 6 and 7).40,41 The variability of crizotinib CL/F was high because of the small sample size.
Discussion
JAVELIN Lung 101 evaluated the safety, efficacy, and PK of avelumab in combination with lorlatinib or crizotinib for patients with ALK-negative or ALK-positive advanced NSCLC, respectively. Most patients had received previous treatment for advanced or metastatic disease. Avelumab plus lorlatinib had a manageable safety profile, whereas avelumab plus crizotinib resulted in DLT in five of 12 patients (42%). The MTD and RP2D for avelumab plus lorlatinib was determined to be avelumab 10 mg/kg IV every 2 weeks plus lorlatinib 100 mg once daily. The MTD for avelumab plus crizotinib was exceeded at the doses administered (10 mg/kg IV every 2 wk and 250 mg twice daily, respectively), and this combination did not proceed to phase 2. Enrollment was stopped early based on the incidence of hepatic toxicity in the avelumab plus crizotinib group and the changing treatment landscape for patients with metastatic ALK-positive NSCLC. Co-administration of avelumab with lorlatinib or crizotinib had no clinically meaningful effect on lorlatinib or crizotinib PK based on comparisons with historical monotherapy data.3,9
Antitumor activity was observed with avelumab plus lorlatinib in patients with ALK-positive tumors and avelumab plus crizotinib in patients with ALK-negative tumors. The ORR for avelumab plus lorlatinib was similar to the ORR reported in a prior study of lorlatinib monotherapy in patients with advanced, ALK-positive NSCLC who had previously received two or more TKIs (52% versus 46%, respectively).10 Median PFS with avelumab plus lorlatinib was comparable with results from a phase 2 study of lorlatinib monotherapy in subgroups of patients with ALK-positive NSCLC who had prior ALK TKI treatment (6.4 versus 6.9–7.3 mo, respectively).11 The ORR for avelumab plus crizotinib in our study (25%) was similar to the ORR reported with avelumab monotherapy in a cohort of patients with untreated advanced NSCLC without ALK or EGFR alterations who were unselected based on PD-L1 status (20%) and higher than the ORR reported with avelumab monotherapy in a cohort of platinum-treated patients (12%).14,42 No meaningful difference was observed in response to treatment based on PD-L1 status. This study was limited by the small sample size, and firm conclusions cannot be drawn.
In all previous clinical studies of ICI plus ALK TKI combination therapy in patients with ALK-positive NSCLC, increased toxicity has been reported compared with single-agent treatment.27, 28, 29, 30 The reason for the higher toxicity in the avelumab plus crizotinib group versus the avelumab plus lorlatinib is unknown but could be due to the lower kinase selectivity of crizotinib versus lorlatinib, which may result in additional effects when crizotinib is administered in combination with an ICI. The high toxicity with avelumab plus crizotinib in this study is consistent with previous reports of ICIs combined with crizotinib. In the CheckMate 370 trial, 38% of patients treated with nivolumab plus crizotinib had grade 3 or higher hepatotoxicity, and the study was terminated early.27 Similarly, with pembrolizumab plus crizotinib, the incidence of grade 3 or higher ALT or AST was high, and the study was terminated early.30 Although combination therapy with nivolumab plus ceritinib or atezolizumab plus alectinib was found to have preliminary antitumor activity, increased toxicity compared with single agents was also observed.28,29
Conclusion
Avelumab plus lorlatinib combination treatment was feasible in patients with ALK-positive advanced NSCLC, but antitumor activity did not seem to be substantially increased compared with previous studies of lorlatinib monotherapy. Avelumab plus crizotinib combination treatment was associated with toxicity issues at the doses evaluated. Results from this study and changes in the treatment landscape in advanced NSCLC do not support further studies of these combinations.
Data Sharing Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the healthcare business of Merck KGaA, Darmstadt, Germany’s (CrossRef Funder ID: 10.13039/100009945) Data Sharing Policy. All requests should be submitted in writing to the healthcare business of Merck KGaA, Darmstadt, Germany’s data sharing portal (https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When the healthcare business of Merck KGaA, Darmstadt, Germany, has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been outlicensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany, will endeavor to gain agreement to share data in response to requests.
CRediT Authorship Contribution Statement
Benjamin J. Solomon: Investigation, Writing—review and editing.
Ibiayi Dagogo-Jack: Investigation, Writing—review and editing.
Se-Hoon Lee: Investigation, Writing—review and editing.
Michael J. Boyer: Investigation, Writing—review and editing.
Suresh S. Ramalingam: Investigation, Writing—review and editing.
Enric Carcereny: Investigation, Writing—review and editing.
Enriqueta Felip: Investigation, Writing—review and editing.
Ji-Youn Han: Investigation, Writing—review and editing.
Toyoaki Hida: Investigation, Writing—review and editing.
Brett G.M. Hughes: Investigation, Writing—review and editing.
Sang-We Kim: Investigation, Writing—review and editing.
Makoto Nishio: Investigation, Writing—review and editing.
Takashi Seto: Investigation, Writing—review and editing.
Tatsuro Okamoto: Investigation, Writing—review and editing.
Xiaoxi Zhang: Data curation, Formal analysis, Visualization, Writing—review and editing,.
Jean-Francois Martini: Data curation, Formal analysis, Visualization, Writing—review and editing,.
Erjian Wang: Data curation, Formal analysis, Visualization, Writing—review and editing.
Steven De Beukelaer: Conceptualization, Data curation, Supervision, Writing—review and editing.
Todd M. Bauers: Investigation, Writing—review and editing.
Disclosure
Dr. Solomon reports providing a consulting or advisory role for Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb, Cancer Council of Victoria, D3 Bio, Janssen, Lilly, the healthcare business of Merck KGaA, Darmstadt, Germany, Pfizer, Roche/Genentech, Takeda, and Thoracic Oncology Group of Australasia; has provided speaker services for Amgen, AstraZeneca, Pfizer, and Roche/Genentech; and has received institutional research funding from BeiGene, Bristol Myers Squibb, Lilly, Novartis, Nuvalent, Pfizer, Roche/Genentech, and Sanofi. Prof. Dagogo-Jack reports providing a consulting or advisory role for AstraZeneca, Bayer, Boehringer Ingelheim, BostonGene, Catalyst Pharmaceuticals, Genentech, Janssen, Novocure, Pfizer, Sanofi, Syros Pharmaceuticals, and Xcovery; has received travel, accommodations, or expenses from Array BioPharma, Creative Educational Concepts, DAVA Oncology, Medscape, OncLive/MJH Life Sciences, Pfizer, The ASCO Post, and Total Health Conferencing; and has received institutional research funding from Array BioPharma, Calithera Biosciences, Genentech, Pfizer, Novartis, and Vivace Therapeutics. Prof. Lee reports providing a consulting or advisory role for AstraZeneca/MedImmune, Bristol Myers Squibb, and Novartis; has received honoraria from AstraZeneca/MedImmune, the healthcare business of Merck KGaA, Darmstadt, Germany, and Roche; and has received travel, accommodations, or expenses from Novartis. Prof. Boyer reports providing a consulting or advisory role for AstraZeneca/MedImmune, Bristol Myers Squibb, and Merck & Co., Kenilworth, NJ; has received institutional research funding from Amgen, Ascentage Pharma, AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Merck & Co., Kenilworth, NJ, OncoMed, Peregrine Pharmaceuticals, Pfizer, and Roche/Genentech; and has received travel, accommodations, or expenses from Boehringer Ingelheim, Bristol Myers Squibb, Merck & Co., Kenilworth, NJ, and Roche/Genentech. Prof. Ramalingam reports providing a consulting or advisory role for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Lilly/ImClone, Roche/Genentech, Takeda, and the healthcare business of Merck KGaA, Darmstadt, Germany. Dr. Carcereny has no relationships to disclose. Prof. Felip reports providing a consulting or advisory role for AbbVie, Amgen, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck & Co., Kenilworth, NJ, Novartis, Pfizer, Puma Biotechnology, Roche, Sanofi, Genzyme, Takeda, and the healthcare business of Merck KGaA, Darmstadt, Germany; has provided speaker services for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Medscape, Merck & Co., Kenilworth, NJ, Novartis, PeerVoice, Pfizer, Prime Oncology, Roche, Springer, Takeda, touchIME, and CME Outfitters; has received research funding from grant for Oncology Innovation, Fundación Merck Salud, Madrid, Spain, an affiliate of Merck KGaA, Darmstadt, Germany; and is an independent member of the board of Grifol. Dr. Han reports providing a consulting or advisory role for Merck & Co., Kenilworth, NJ; has received honoraria from Novartis; and has received institutional research funding from Roche. Dr. Hida has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharma, Clovis Oncology, Kissei Pharmaceutical, Lilly, Merck & Co., Kenilworth, NJ, Novartis, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical; and has received institutional research funding from AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharma, Clovis Oncology Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Ignyta, Kissei Pharmaceutical, Kyowa Hakko Kirin, Merck & Co., Kenilworth, NJ, Novartis, Ono Pharmaceutical, Pfizer, Servier, Taiho Pharmaceutical, Takeda, and the healthcare business of Merck KGaA, Darmstadt, Germany. Dr. Hughes reports providing a consulting or advisory role for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Pfizer, Merck & Co., Kenilworth, NJ, and Roche. Dr. Kim has no relationships to disclose. Dr. Nishio has received lecture fees from Chugai Pharmaceutical, Pfizer, Novartis, and Takeda. Dr. Seto has received honoraria from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharma, Kissei Pharmaceutical, Kyowa Hakko Kirin, Lilly Japan, Merck & Co., Kenilworth, NJ, Nippon Kayaku, Ono Pharmaceutical, Pfizer, Roche Singapore, Taiho Pharmaceutical, Takeda, and Yakult Honsha; and has received institutional research funding from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Boehringer Ingelheim, Chugai Pharma, Daiichi Sankyo, Eisai, Kissei Pharmaceutical, Lilly, Merck & Co., Kenilworth, NJ, Novartis, Pfizer, and the healthcare business of Merck KGaA, Darmstadt, Germany. Dr. Okamoto has received honoraria from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Johnson & Johnson, Merck & Co., Kenilworth, NJ, Boehringer Ingelheim, Nippon Kayaku, Novartis, Ono Pharmaceutical, and Taiho Pharmaceutical; and has received institutional research funding from AnHeart Therapeutics, AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Covidien Japan, Daiichi Sankyo, Lilly, KM Biologics, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co., Kenilworth, NJ, Boehringer Ingelheim, Nippon Kayaku, Novartis, Pfizer, and Taiho Pharmaceutical. Dr. Zhang is an employee of Pfizer and owns stock and has other ownership interests in Pfizer. Dr. Martini is an employee of Pfizer and owns stock and has other ownership interests in Pfizer. Dr. Wang was an employee of Pfizer at the time this study was conducted. Dr. De Beukelaer was an employee of Pfizer at the time this study was conducted and owns stock and has other ownership interests in Pfizer. Dr. Bauer reports providing a consulting or advisory role for AstraZeneca, Bayer, Lilly, and Pfizer; has provided speaker services for Bayer, Bristol Myers Squibb, Lilly, and Pfizer; and has received institutional research funding from AstraZeneca, Bayer, Bristol Myers Squibb, Lilly, and Pfizer.
Acknowledgments
This trial was sponsored by Pfizer and was previously conducted under an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945), and Pfizer. Medical writing support was provided by Hiba Al-Ashtal of Nucleus Global and was funded by the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer. The authors thank the patients and their families, investigators, co-investigators, and the study teams at each of the participating centers.
Footnotes
Cite this article as: Solomon BJ, Dagogo-Jack I, Lee S-H, et al. Avelumab in combination with lorlatinib or crizotinib in patients with previously treated advanced NSCLC: phase 1b/2 results from the JAVELIN lung 101 trial. JTO Clin Res Rep 2024;5:100685
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100685.
Supplementary Data
References
- 1.NCCN Clinical Practice Guidelines in Oncology . 2024. Non-small Cell Lung Cancer. Version 5. [Google Scholar]
- 2.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 3.Xalkori (crizotinib) Pfizer; New York, NY: 2023. Prescribing Information. [Google Scholar]
- 4.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagogo-Jack I., Shaw A.T. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27:iii42–iii50. doi: 10.1093/annonc/mdw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alecensa (alectinib) Genentech Inc; South San Francisco, CA: 2024. Prescribing Information. [Google Scholar]
- 7.Alunbrig (brigatinib) Takeda Pharmaceuticals; Tokyo, Japan: 2022. Prescribing Information. [Google Scholar]
- 8.Zykadia (ceritinib) Novartis Pharmaceuticals; Basel, Switzerland: 2022. Prescribing Information. [Google Scholar]
- 9.Lorbrena (lorlatinib) Pfizer; New York, NY: 2023. Prescribing Information. [Google Scholar]
- 10.Shaw A.T., Felip E., Bauer T.M., et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon B.J., Besse B., Bauer T.M., et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 12.Bavencio (avelumab) EMD Serono; Rockland, MA: 2024. Prescribing Information. [Google Scholar]
- 13.2024. Bavencio (avelumab). Summary of product characteristics. Merck Europe B.V., Amsterdam, The Netherlands, an affiliate of Merck KGaA, Darmstadt, Germany. [Google Scholar]
- 14.Verschraegen C.F., Jerusalem G., McClay E.F., et al. Efficacy and safety of first-line avelumab in patients with advanced non-small cell lung cancer: results from a phase Ib cohort of the JAVELIN Solid Tumor study. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlesi F., Vansteenkiste J., Spigel D., et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19:1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 16.Park K., Özgüroğlu M., Vansteenkiste J., et al. Avelumab versus docetaxel in patients with platinum-treated advanced NSCLC: 2-year follow-up from the JAVELIN Lung 200 phase 3 trial. J Thorac Oncol. 2021;16:1369–1378. doi: 10.1016/j.jtho.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Reck M., Barlesi F., Yang J.C., et al. Avelumab versus platinum-based doublet chemotherapy as first-line treatment for patients with high-expression programmed death-ligand 1-positive metastatic NSCLC: primary analysis from the phase 3 JAVELIN Lung 100 trial. J Thorac Oncol. 2024;19:297–313. doi: 10.1016/j.jtho.2023.09.1445. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L., Liu J. Immunological effect of tyrosine kinase inhibitors on the tumor immune environment in non-small cell lung cancer. Oncol Lett. 2022;23:165. doi: 10.3892/ol.2022.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ota K., Azuma K., Kawahara A., et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21:4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 20.Koh J., Jang J.Y., Keam B., et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1α and STAT3. Oncoimmunology. 2015;5 doi: 10.1080/2162402X.2015.1108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang G.C., Yang T.Y., Chen K.C., et al. ALK variants, PD-L1 expression, and their association with outcomes in ALK-positive NSCLC patients. Sci Rep. 2020;10 doi: 10.1038/s41598-020-78152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong S., Chen N., Fang W., et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2015;5 doi: 10.1080/2162402X.2015.1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng H., Zeltsman M., Zauderer M.G., Eguchi T., Vaghjiani R.G., Adusumilli P.S. Chemotherapy-induced immunomodulation in non-small-cell lung cancer: a rationale for combination chemoimmunotherapy. Immunotherapy. 2017;9:913–927. doi: 10.2217/imt-2017-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Y., Wang Y., Zeng D., et al. Comprehensive analyses reveal TKI-induced remodeling of the tumor immune microenvironment in EGFR/ALK-positive non-small-cell lung cancer. Oncoimmunology. 2021;10 doi: 10.1080/2162402X.2021.1951019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vladimer G.I., Snijder B., Krall N., et al. Global survey of the immunomodulatory potential of common drugs. Nat Chem Biol. 2017;13:681–690. doi: 10.1038/nchembio.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P., Shaffer D.R., Alvarez Arias D.A., et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spigel D.R., Reynolds C., Waterhouse D., et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370) J Thorac Oncol. 2018;13:682–688. doi: 10.1016/j.jtho.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Felip E., de Braud F.G., Maur M., et al. Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: results of an open-label, multicenter, phase 1b study. J Thorac Oncol. 2020;15:392–403. doi: 10.1016/j.jtho.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Kim D.W., Gadgeel S., Gettinger S.N., et al. Brief report: safety and antitumor activity of alectinib plus atezolizumab from a phase 1b study in advanced ALK-positive NSCLC. JTO Clin Res Rep. 2022;3 doi: 10.1016/j.jtocrr.2022.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel S.P., Pakkala S., Pennell N.A., et al. Phase Ib study of crizotinib plus pembrolizumab in patients with previously untreated advanced non-small cell lung cancer with ALK translocation. Oncologist. 2020;25:e562–e1012. doi: 10.1634/theoncologist.2020-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon B.J., Mok T., Kim D.W., et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 34.Shaw A.T., Kim T.M., Crinò L., et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 35.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 37.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 38.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 39.Dempke W.C.M., Fenchel K. Has programmed cell death ligand-1 MET an accomplice in non-small cell lung cancer?-a narrative review. Transl Lung Cancer Res. 2021;10:2667–2682. doi: 10.21037/tlcr-21-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson T.R., Tan W., Goulet L., et al. Metabolism, excretion and pharmacokinetics of [14C]crizotinib following oral administration to healthy subjects. Xenobiotica. 2015;45:45–59. doi: 10.3109/00498254.2014.941964. [DOI] [PubMed] [Google Scholar]
- 41.Xu H., O’Gorman M., Boutros T., et al. Evaluation of crizotinib absolute bioavailability, the bioequivalence of three oral formulations, and the effect of food on crizotinib pharmacokinetics in healthy subjects. J Clin Pharmacol. 2015;55:104–113. doi: 10.1002/jcph.356. [DOI] [PubMed] [Google Scholar]
- 42.Gulley J.L., Rajan A., Spigel D.R., et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18:599–610. doi: 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.