Figure 1. SCA identifies the LPTYPFx5W motif as a candidate fusion point for chimeric trans-AT PKSs.
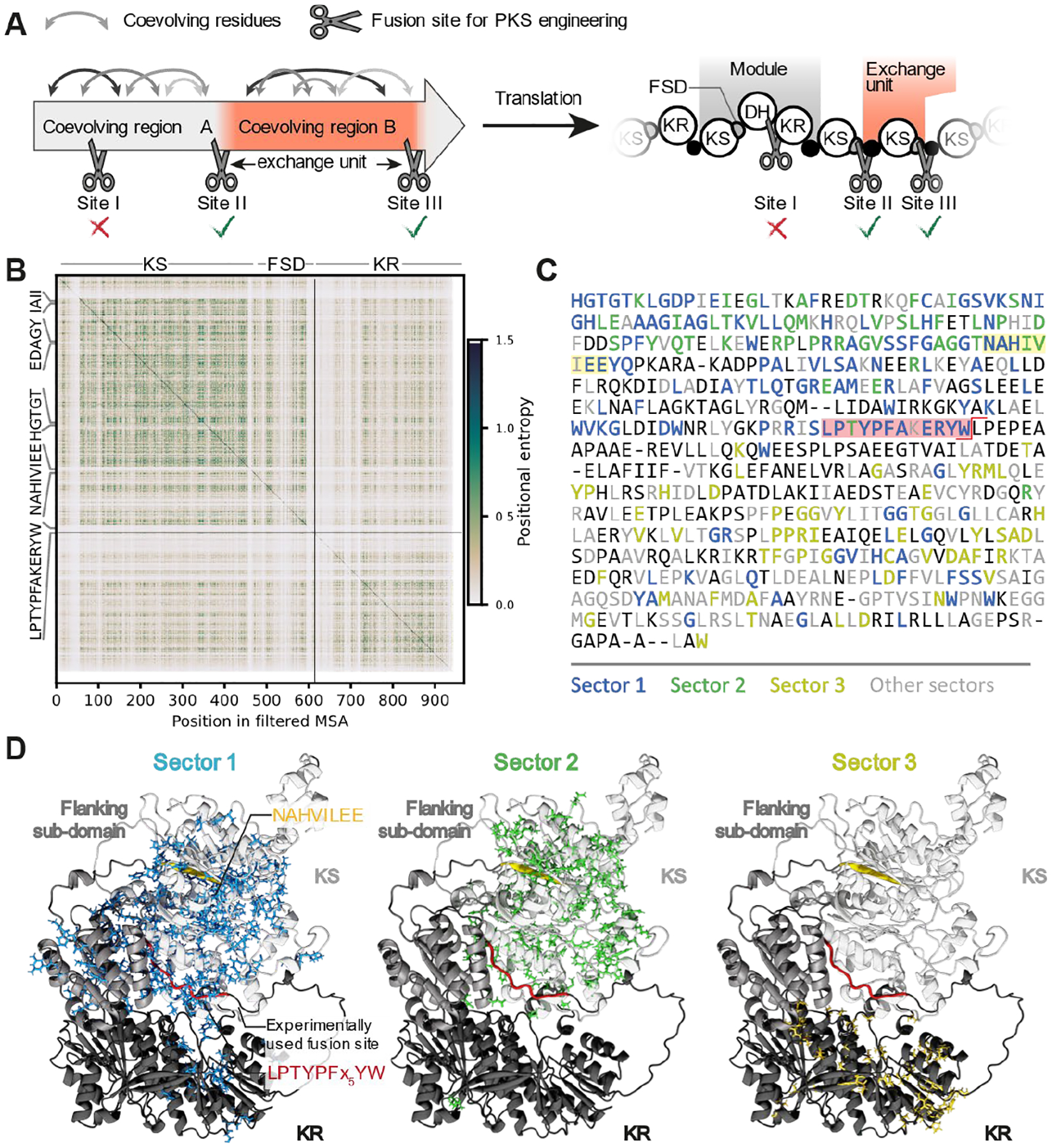
(A) Engineering within regions of coevolving residues is hypothesized to perturb important amino acid interactions, yielding non-functional chimeras (site I). In contrast, engineering at boundaries between regions of coevolving residues minimizes such perturbations and might lead to functional chimeras (sites II and III). At the protein level, trans-AT PKS exchange units span between the C-terminal boundaries of the FSDs, which slightly contrasts with the commonly used PKS module boundaries, spanning from KS to ACP. (B) Covariance matrix of Clustal-aligned (53) multiple sequence alignments of the KS-FSD-KR tridomain, showing the positional entropy obtained from SCA for amino acid residue pairs of the tridomain. The position of conserved IAII, HGTGT, NAHVILEE, and LPTYPFx5W motifs are indicated on the side. (C) Consensus sequence of the C-terminal part of the KS-FSD-KR tridomain obtained from the conservation-filtered sequence alignment used for SCA. Residues are color-coded according to SCA sectors. Residues not assigned to sectors are black. The NAHVILEE motif and the experimentally used engineering site downstream of the LPTYPFx5W motif is indicated with the yellow and red shades, respectively. (D) 3D visualizations of sector 1 (left), sector 2 (middle), and sector 3 (right) on an alphaFold2-generated (54) model of the OocR KS-FSD-KR tridomain. Colors correspond to residue colors in panel B.
