Fig. 1 |. Fundamental aspects of mitochondrial genetics.
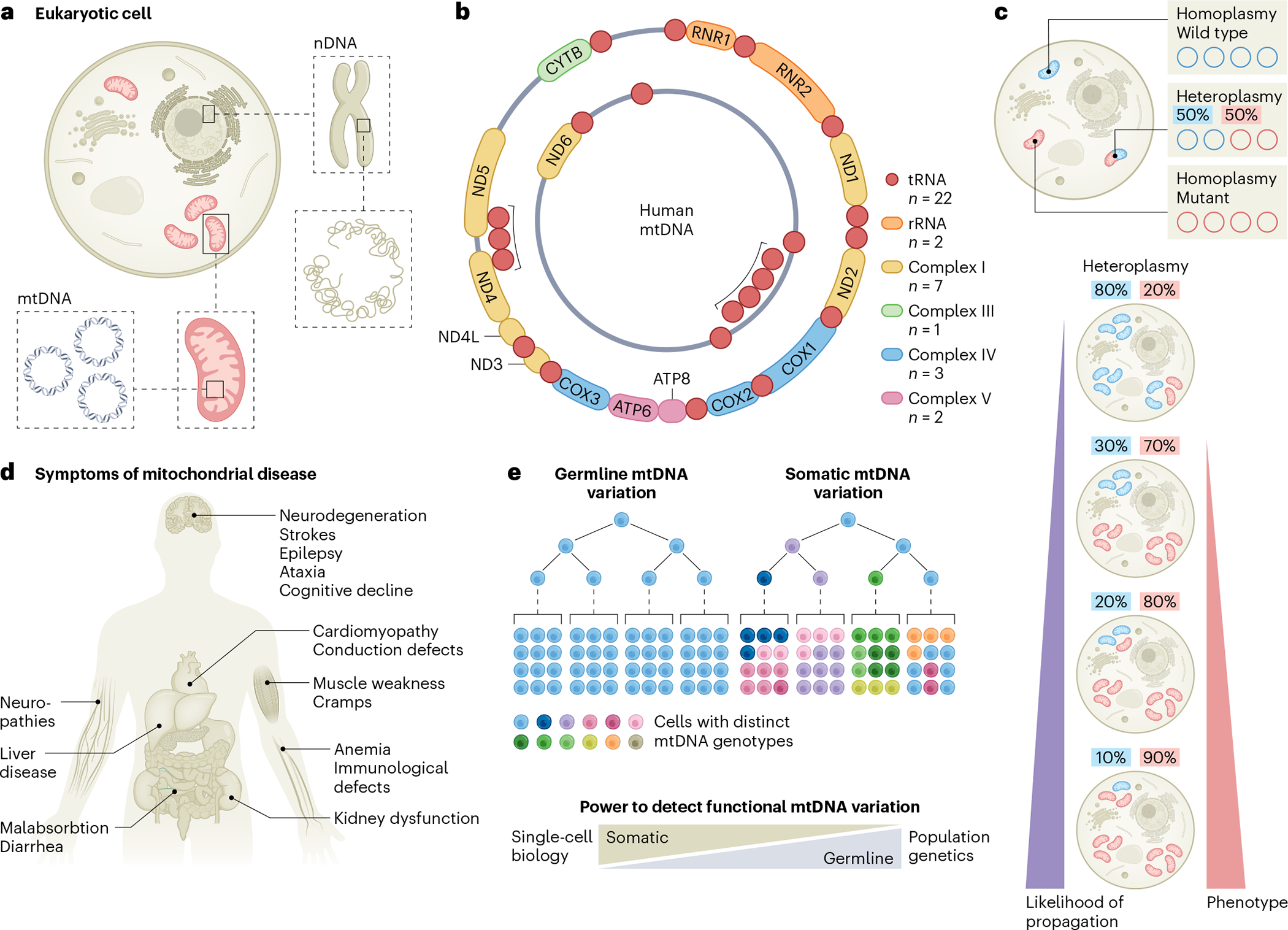
a, Schematic illustrating the physical separation of the set of linear chromosomes representing the nuclear genome and mtDNA located in mitochondria. b, Schematic of the double-stranded, circular mitochondrial genome encoding 22 tRNAs and 2 rRNAs alongside 13 protein-coding genes, which contribute to indicated complexes of the respiratory chain (I, III, IV and V; color-coded). c, Individual cells have multiple mitochondria with potentially multiple copies of mtDNA. Each copy may have unique mutations leading to a state of heteroplasmy. As such, individual mitochondria, as well as cells, may have different allele frequencies of any given mutation. While low-allele-frequency variants may be more dynamic, also given the natural turnover of mitochondria and mtDNA within, individual mtDNA mutations may reach high allele frequencies, even up to homoplasmy owing to, for example, genetic drift. With increasing heteroplasmy, the likelihood of their stable propagation across cell divisions is increased, serving as natural genetic barcodes of clonality. Pathogenic mtDNA mutations may show variable phenotypic effects as a function of heteroplasmy, with a high mutational burden leading to pronounced cellular pathology and human disease. d, Various organ systems may be affected in patients with mitochondrial disorders. e, Germline mtDNA variants, including mtDNA haplotypes, exhibit largely no variation among cells of a single individual. By contrast, a major fraction of cells may develop unique mitochondrial genomes due to somatic mutations that may arise with age. Emerging single-cell omics approaches now enable a more sensitive detection of rare and functionally relevant mtDNA mutations compared to widely used population-based genetic approaches. Panel b adapted from ref. 78, Springer Nature Limited.
