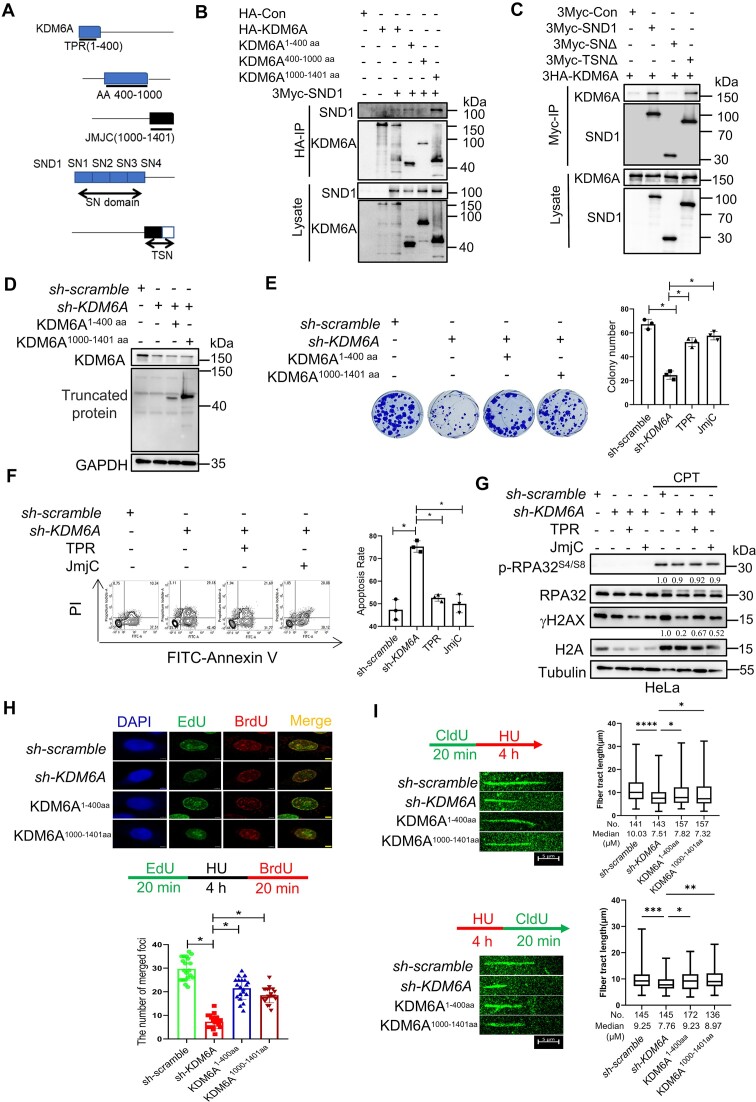
Figure 5.
The TPR-JmjC domain of KDM6A interacted with SN domain of SND1 and involved in the stability and restart of replication forks. (A) Schematic diagram shows the construction strategy of KDM6A and SND1 mutants. (B, C) The domain of TPR and JmjC in KDM6A and SN domain in SND1 contribute to KDM6A–SND1 interaction. After co-transfection of KDM6A and SND1, KDM6A (B) or SND1 (C) was immunoprecipitated in HEK293T cells using specific tag antibodies, followed by western blot with indicated antibodies. (D–F) Complement of KDM6A TPR or JmjC domain partially rescue the viability of HeLa cells lacking endogenous KDM6A in response to CPT treatment. (G) Western blot for phospho-RPA32S4/S8 and γH2AX in HeLa cells. Lysate from cells lacking endogenous KDM6A in combination of KDM6A TPR or JmjC domain complement was subjected to western blot. Quantification of phospho-RPA32S4/S8 and γH2AX protein were determined against RPA32 and γH2AX expression respectively. (H) BrdU and EdU were incorporated into nascent DNA for immunofluorescence observation. The histogram showed the number of replication foci formation in indicated cells. (I) DNA fiber assay for exploring the influence of KDM6A on the progression (upper panel) and restart (lower panel) of DNA replication forks. The histogram showed the alteration in the length of nascent DNA in HeLa. All experiments were independently repeated three times, *P < 0.05. P values were determined using a paired Student's t test.