Abstract
Background
Uricosuric agents have long been used in the treatment of gout but there is little evidence regarding their benefit and safety in this condition.
Objectives
To assess the benefits and harms of uricosuric medications in the treatment of chronic gout.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 4, 2013), Ovid MEDLINE and Ovid EMBASE for studies to the 13 May 2013. We also searched the World Health Organization Clinical Trials Registry, ClinicalTrials.gov and the 2011 to 2012 American College of Rheumatology and European League against Rheumatism abstracts. WE considered black box warnings and searched drug safety databases to identify and describe rare adverse events.
Selection criteria
We considered all randomised controlled trials (RCTs) or quasi‐randomised controlled trials (controlled clinical trials (CCTs)) that compared uricosuric medications (benzbromarone, probenecid or sulphinpyrazone) alone or in combination with another therapy (placebo or other active uric acid‐lowering medication, or non‐pharmacological treatment) in adults with chronic gout for inclusion.
Data collection and analysis
Two review authors independently selected the studies for inclusion, extracted data and performed a risk of bias assessment. Main outcomes were frequency of acute gout attacks, serum urate normalisation, study participant withdrawal due to adverse events, total adverse events, pain reduction, function and tophus regression.
Main results
The search identified four RCTs and one CCT that evaluated the benefit and safety of uricosurics for gout. One study (65 participants) compared benzbromarone with allopurinol for a duration of four months; one compared benzbromarone with allopurinol (36 participants) for a duration of nine to 24 months; one study (62 participants) compared benzbromarone with probenecid for two months and one study (74 participants) compared benzbromarone with probenecid. One study (37 participants) compared allopurinol with probenecid. No study was completely free from bias.
Low‐quality evidence from one study (55 participants) comparing benzbromarone with allopurinol indicated uncertain effects in terms of frequency of acute gout attacks (4% with benzbromarone versus 0% with allopurinol; risk ratio (RR) 3.58, 95% confidence interval (CI) 0.15 to 84.13), while moderate‐quality evidence from two studies (101 participants; treated for four to nine months) indicated similar proportions of participants achieving serum urate normalisation (73.9% with benzbromarone versus 60% with allopurinol; pooled RR 1.27, 95% CI 0.90 to 1.79). Low‐quality evidence indicated uncertain differences in withdrawals due to adverse events (7.1% with benzbromarone versus 6.1% with allopurinol; pooled RR 1.25, 95% CI 0.28 to 5.62), and total adverse events (20% with benzbromarone versus 6.7% with allopurinol; RR 3.00, 95% CI 0.64 to 14.16). The study did not measure pain reduction, function and tophus regression.
When comparing benzbromarone with probenecid, there was moderate‐quality evidence based on one study (62 participants) that participants taking benzbromarone were more likely to achieve serum urate normalisation after two months (81.5% with benzbromarone versus 57.1% with probenecid; RR 1.43, 95% CI 1.02 to 2.00). This indicated that when compared with probenecid, five participants needed to be treated with benzbromarone in order to have one additional person achieve serum urate normalisation (number needed to treat for an additional beneficial outcome (NNTB) 5). However, the second study reported no difference in the absolute decrease in serum urate between these groups after 12 weeks. Low‐quality evidence from two studies (129 participants) indicated uncertain differences between treatments in the frequency of acute gout attacks (6.3% with benzbromarone versus 10.6% with probenecid; pooled RR 0.73, 95% CI 0.09 to 5.83); fewer withdrawals due to adverse events with benzbromarone (2% with benzbromarone versus 17% with probenecid; pooled RR 0.15, 95% CI 0.03 to 0.79, NNTB 7) and fewer total adverse events (21% with benzbromarone versus 47% with probenecid; pooled RR 0.43, 95% CI 0.25 to 0.74; NNTB 4). The studies did not measure pain reduction, function and tophus regression.
Low‐quality evidence based on one small CCT (37 participants) indicated uncertainty around the difference in the incidence of acute gout attacks between probenecid and allopurinol after 18 to 20 months' treatment (53% with probenecid versus 55% with allopurinol; RR 0.96, 95% CI 0.53 to 1.75). The study did not measure or report the proportion achieving serum urate normalisation, pain reduction, function, tophus regression, withdrawal due to adverse events and total adverse events.
Authors' conclusions
There was moderate‐quality evidence that there is probably no important difference between benzbromarone and allopurinol at achieving serum urate normalisation, but that benzbromarone is probably more successful than probenecid at achieving serum urate normalisation in people with gout. There is some uncertainty around the effect estimates, based on low‐quality evidence from only one or two trials, on the number of acute gout attacks, the number of withdrawals due to adverse events or the total number of participants experiencing adverse events when comparing benzbromarone with allopurinol. However, when compared with probenecid, benzbromarone resulted in fewer withdrawals due to adverse events and fewer participants experiencing adverse events. Low‐quality evidence from one small study indicated uncertain effects in the incidence of acute gout attacks when comparing probenecid with allopurinol therapy. We downgraded the evidence because of a possible risk of performance and other biases and imprecision.
Keywords: Adult, Aged, Female, Humans, Male, Middle Aged, Allopurinol, Allopurinol/therapeutic use, Benzbromarone, Benzbromarone/therapeutic use, Chronic Disease, Controlled Clinical Trials as Topic, Gout, Gout/blood, Gout/drug therapy, Probenecid, Probenecid/therapeutic use, Randomized Controlled Trials as Topic, Uric Acid, Uric Acid/blood, Uricosuric Agents, Uricosuric Agents/adverse effects, Uricosuric Agents/therapeutic use
Plain language summary
Uricosuric medications for chronic gout
Background ‐ what is chronic gout and what are uricosuric medications?
Chronic gout is a type of inflammatory arthritis caused by high levels of uric acid in the blood leading to crystal formation in the joints and repeated attacks of acute gout. Treatment of chronic gout can make acute gout attacks less likely.
Uricosuric medications work to lower blood uric acid levels by increasing the amount of uric acid that is eliminated via the kidneys.
Study characteristics
This summary of a Cochrane review presents what we know from research about the effect of uricosuric medications for treating chronic gout. After searching for all relevant studies to May 2013, we found five studies. Most participants in these studies were male (81% to 100%), aged between 50 and 70 years and did not have significant kidney or liver disease. Two trials compared benzbromarone with allopurinol, two trials compared benzbromarone with probenecid and one trial compared allopurinol with probenecid. We found no studies comparing uricosuric medications with placebo, or with newer urate‐lowering therapies such as febuxostat or pegloticase. We restricted reporting of results here to benzbromarone versus allopurinol, as allopurinol is a commonly used first‐line treatment for gout.
Key results ‐ what happens to people with chronic gout who take uricosuric medications
Benzbromarone compared with allopurinol:
Acute gout attacks:
‐ 4 fewer people out of 100 had acute gout attacks after four months' treatment with allopurinol compared with benzbromarone.
‐ 4 people out of 100 on benzbromarone had acute gout attacks.
‐ 0 people out of 100 on allopurinol had acute gout attacks.
Proportion achieving normal serum urate:
‐ 17 more people out of 100 achieved a normal serum urate level after for months on benzbromarone compared with allopurinol.
‐ 76 people out of 100 on benzbromarone achieved a normal serum urate level after four months.
‐ 60 people out of 100 on allopurinol achieved a normal serum urate level after four months.
Withdrawal due to side effects:
‐ 1 more person out of 100 stopped benzbromarone due to side effects compared with allopurinol.
‐ 8 people out of 100 stopped benzbromarone due to side effects.
‐ 6 people out of 100 stopped allopurinol due to side effects.
The trials did not report pain, function and tophus regression.
Quality of the evidence
Low‐quality evidence indicated that there was no important difference in acute gout attacks, or in the number of people who had to stop the medication due to a side effect between benzbromarone and allopurinol. Moderate‐quality evidence from two studies showed that both medications reduced uric acid levels to a similar degree. Benzbromarone was no more likely than probenecid to result in acute gout attacks, but may result in fewer withdrawals due to side effects (low‐quality evidence), and is probably more likely to reduce uric acid levels to a normal level (moderate‐quality evidence). The trials did not measure pain, function and tophus regression.
Low‐quality evidence from a single study indicated that probenecid was no more likely to result in acute gout attacks than allopurinol. Further research is highly likely to impact on this estimate. This study did not measure proportion of people achieving a normal serum urate level, pain reduction, function, tophus regression or the number of people stopping the medication due to side effects.
Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimates.
We found no studies that looked at sulphinpyrazone in treating chronic gout. We do not have information about the effects of uricosuric medications in particular groups of patients such as people with impaired kidney function or with different levels of uric acid. Possible side effects of uricosuric medications may include rash, stomach upset, flushing, dizziness, headache or acute gout attacks. Rare complications may include liver failure, kidney stones, anaemia or allergic reaction.
Summary of findings
Summary of findings for the main comparison. Benzbromarone compared with allopurinol for chronic gout.
| Benzbromarone compared with allopurinol for chronic gout | ||||||
| Patient or population: people with chronic gout Settings: Intervention: benzbromarone Comparison: allopurinol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allopurinol | Benzbromarone | |||||
| Acute gout attacks Follow‐up: mean 4 months | Study population | RR 3.58 (0.15 to 84.13) | 55 (1 study) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 4% more attacks with benzbromarone (6% fewer to 14% more) Relative change: 258% worse with benzbromarone (85% better to 8313% worse) NNT ‐ n/a3 |
|
| 11 per 1000 | 40 per 1000 (0 to 140) | |||||
| Serum urate normalisation Follow‐up: 4‐9 months | Study population | RR 1.27 (0.9 to 1.79) | 101 (2 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference: 17% more achieving serum urate normalisation with benzbromarone (10% fewer to 45% more) Relative change: 27% better with benzbromarone (10% worse to 79% better) NNT ‐ n/a3 |
|
| 600 per 1000 | 762 per 1000 (540 to 1000) | |||||
| Withdrawal due to adverse events Follow‐up: median 4‐9 months | Study population | RR 1.25 (0.28 to 5.62) | 91 (2 studies) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 1% fewer with benzbromarone (11% fewer to 10% more) Relative change: 25% better with benzbromarone (72% worse to 462% better) NNT ‐ n/a3 |
|
| 61 per 1000 | 77 per 1000 (17 to 344) | |||||
| Total adverse events Follow‐up: mean 4 months | Study population | RR 3 (0.64 to 14.16) | 55 (1 study) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 13% more with benzbromarone (5% fewer to 31% more) Relative change: 200% worse (36% worse to 1316% better) NNT ‐ n/a3 |
|
| 67 per 1000 | 200 per 1000 (43 to 944) | |||||
| Pain reduction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Function ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Tophus regression ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Open‐label study with possible performance and detection bias and unclear risk related to possible attrition bias. 2 Small study (65 participants). Few events resulting in a wide CI. 3 Number needed to treat an additional beneficial outcome (NNTB) or harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (Cochrane Musculoskeletal Group editorial office).
Summary of findings 2. Benzbromarone compared with probenecid for chronic gout.
| Benzbromarone compared with probenecid for chronic gout | ||||||
| Patient or population: people with chronic gout Settings: Intervention: benzbromarone Comparison: probenecid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Probenecid | Benzbromarone | |||||
| Acute gout attacks Follow‐up: 2‐3 months | 106 per 1000 | 77 per 1000 (10 to 618) | RR 0.73 (0.09 to 5.83) | 129 (2 studies) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 3% fewer attacks with benzbromarone (21% fewer to 14% more) Relative change: 27% better with benzbromarone (91% worse to 483% better) NNT ‐ n/a5 |
| Serum urate normalisation ≤ 5 mg/dL (≤ 0.3 mmol/L) Follow‐up: mean 2 months | 571 per 1000 | 817 per 1000 (583 to 1000) | RR 1.43 (1.02 to 2) | 62 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference: 24% more reached serum urate normalisation with benzbromarone (2% more to 46% more) Relative change: 43% improvement with benzbromarone (2% improvement to 100% improvement) NNTB 5 |
| Withdrawal due to adverse events Follow‐up: mean 2.5 months | 167 per 1000 | 25 per 1000 (5 to 132) | RR 0.15 (0.03 to 0.79) | 129 (2 studies) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 13% fewer with benzbromarone (27% fewer to 0%) Relative change: 85% better with benzbromarone (21% better to 97% better) NNTB 7 |
| Total adverse events Follow‐up: mean 2.5 months | 470 per 1000 | 202 per 1000 (117 to 348) | RR 0.43 (0.25 to 0.74) | 129 (2 studies) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 27% fewer with benzbromarone (42% fewer to 11% fewer) Relative change: 57% better with benzbromarone (26% better to 75% better) NNTB 4 |
| Pain Reduction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Function ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Tophus regression ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Open‐label studies with possible performance and detection bias and unclear risk related to possible attrition bias. 2 Small studies (136 participants). Few events resulting in wide CI.
3 Number needed to treat for an additional beneficial outcome (NNTB) or harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (Cochrane Musculoskeletal Group editorial office).
Summary of findings 3. Probenecid compared with allopurinol for chronic gout.
| Probenecid compared with allopurinol for chronic gout | ||||||
| Patient or population: people with chronic gout Settings: Intervention: probenecid Comparison: allopurinol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allopurinol | Probenecid | |||||
| Acute gout attacks Follow‐up: median 19.1 months | Study population | RR 0.96 (0.53 to 1.75) | 37 (1 study) | ⊕⊕⊝⊝ low1,2 | Absolute risk difference: 2% fewer attacks for probenecid (34% fewer to 30% more) Relative change: 4% better for probenecid (47% better to 75% worse) NNT ‐ n/a3 |
|
| 550 per 1000 | 528 per 1000 (291 to 962) | |||||
| Serum urate normalisation ≤ 5 mg/dL (≤ 0.3 mmol/L) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Withdrawal due to adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Total adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Pain reduction ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Function ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Tophus regression ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Open‐label study with possible performance and detection bias and unclear risk related to possible attrition bias. 2 Small study (37 participants). Few events resulting in wide CI.
3 Number needed to treat for an additional beneficial outcome (NNTB) or harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (Cochrane Musculoskeletal Group editorial office).
Background
Description of the condition
Gout is an inflammatory arthritis characterised by the presence of monosodium urate crystals in joints and other tissues. While the initial manifestation of gout is usually an acute arthritis, the presence of urate crystals can lead to tophus formation and chronic synovitis, which can result in a destructive arthropathy. Epidemiological studies have found gout to be the most common inflammatory arthritis in adult men (estimated prevalence between 1% and 2% of adult males in western countries (Smith 2010)). Further studies indicate that the prevalence of gout is steadily increasing both in men and women with the prevalence of gout in adults in the US being 3.9% (Zhu 2011). The most significant risk factor for the development of gout is hyperuricaemia (serum uric acid 6.8 mg/dL or greater (0.41 mmol/L or greater)), and the incidence of gout increases with an increasing degree of hyperuricaemia (Campion 1987). Lowering serum uric acid levels decreases the frequency of acute gout attacks (Shoji 2004), and this has formed the basis for the long‐term therapy of gout.
Description of the intervention
Medications that lower uric acid levels have been the mainstay of gout therapy. Uricosuric medications act by increasing renal excretion of uric acid, thereby decreasing serum urate levels. Uricosuric medications were the first available gout treatments followed shortly thereafter by allopurinol, a xanthine oxidase inhibitor that acts to decrease uric acid production. There are three primary uricosuric medications used in gout treatment: probenecid, sulphinpyrazone and benzbromarone. More recently, febuxostat, a non‐purine analogue xanthine oxidase inhibitor, has become available to treat gout.
The availability of different uricosuric medications varies between countries. In 2003, the primary manufacturer withdrew benzbromarone due to concerns regarding case reports of possible hepatotoxicity (Hautekeete 1995). However, it remains available in several European countries due to production by several different manufacturers (Lee 2008).
How the intervention might work
Probenecid, benzbromarone and sulphinpyrazone have all inhibit both the human organic anion transporter 4 (hOAT4) as well as URAT 1, a urate‐anion exchanger localised in the renal proximal tubule (Hagos 2007; Shin 2011). This inhibition prevents uric acid reabsorption by the kidneys thereby lowering of the plasma uric acid level. In turn, lowering the serum uric acid level allows dissolution of uric acid crystals from the tissues thereby reducing the body's burden of uric acid. While lowering uric acid levels results in a long‐term reduction in acute gout flares, there is an initial and temporary increase in acute gout attacks when any uric acid‐lowering therapy is initiated due to mobilisation of the uric acid stores. People are often provided with concurrent therapy such as non‐steroidal anti‐inflammatory drugs (NSAID) or colchicine when initiating uric acid‐lowering therapy to lower this risk of acute gout attacks.
Why it is important to do this review
Uricosuric medications have been used since the 1960s and continue to be used in the treatment of gout. By systematically reviewing the literature regarding the benefit and safety of these medications, this review will provide an evidence base for the use of these medications in people with gout. Moreover, with the advent of novel uric acid‐lowering therapies, this review will provide context with which to compare these medications with other uric acid‐lowering medications and thus allow for better evidence‐based clinical treatment decisions.
Objectives
To assess the benefits and harms of uricosuric medications in the treatment of chronic gout.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) or quasi‐randomised controlled trials (controlled clinical trials (CCTs)) that compared uricosuric medications (alone or in combination) with another therapy (placebo or other active uric acid‐lowering medication, or non‐pharmacological treatment) in people with chronic gout for inclusion. We considered only trials published as full articles or available as a full trial report for inclusion.
We excluded studies of uricosuric medications in chronic gout that did not have the outcome measures of interest.
Types of participants
We searched for trials that included adults (aged 18 years or older) with either author‐defined gout or fulfilling gout diagnostic criteria (e.g. Preliminary American College of Rheumatology (ACR) criteria (Wallace 1977)). We excluded populations that included a mix of people with chronic gout and asymptomatic hyperuricaemia unless results for the chronic gout population could be separated out for analysis.
Types of interventions
We included all trials that evaluated a uricosuric medication (probenecid, sulphinpyrazone or benzbromarone) in any dose or dosing interval.
Comparators could be:
placebo;
no treatment;
allopurinol;
febuxostat;
a different uricosuric medication;
non‐pharmacological therapy.
Types of outcome measures
We based the outcome measures on the 2010 Outcome Measures in Rheumatology Meeting (OMERACT 10) gout report recommended outcome domains for chronic gout (Schumacher 2009; Singh 2011).
Major outcomes
Frequency of acute gout attacks.
Serum urate normalisation as measured by per cent change in uric acid from baseline, absolute change in uric acid from baseline (mg/dL or μmol/L) or per cent of participants achieving a normal serum urate (e.g. less than 6 mg/dL or less than 360 μmol/L).
Pain, for example as measured on the visual analogue scale (VAS), numeric rating scale (NRS), Likert scales or qualitative scales.
Function (i.e. activity limitation), for example as measured by the Health Assessment Questionnaire Disability Index (HAQ‐DI), 36‐item Short Form (SF‐36) Physical Component or other validated gout‐specific function measures.
Tophus regression using physical measurement techniques (e.g. Vernier callipers) or ultrasound‐guided measurements (Dalbeth 2011).
Study participant withdrawal due to adverse events (AE).
Total AEs as measured by the total number and description of these events.
Minor outcomes
Health‐related quality of life (e.g. SF‐36).
Participant global assessment of treatment success.
We extracted data from all time points and combined them into short term (less than six weeks) and long term (greater than six weeks). If more than one time point was reported within the subgroup (e.g. at one‐month and two‐month follow‐up), we extracted only the later time point (i.e. two months).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 4, 2013 (Appendix 1);
Ovid MEDLINE 1950 to 13 May 2013 (Appendix 2);
EMBASE 1980 to 13 May 2013 (Appendix 3).
There were no limitations based on language, year of publication or type of publication.
Searching other resources
We inspected the reference lists of included articles and systematic reviews for additional trials and searched the World Health Organization (WHO) trial registry (www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov) to 7 July 2014 for additional trials.
We searched the abstracts from the two major international rheumatology scientific meetings ‐ the ACR and the European League Against Rheumatism (EULAR) ‐ for the years 2010 to 2012 to identify unpublished trials. We included unpublished manuscripts as studies awaiting classification. For rare serious adverse events (SAEs), we also searched black box warnings and regulatory agency sources.
Medicine and Health Care products Regulatory Agency, 'Drug Safety Update' (www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/index.htm).
Australian Database of Adverse Event Notifications (www.tga.gov.au/safety/index.htm).
MedWatch: the Food and Drug Administration (FDA) Safety Information and Adverse Event Reporting Program (US) ‐ Adverse Event Reporting System (www.fda.gov/Safety/MedWatch/default.htm).
European Public Assessment Reports from the European Medicines Evaluation Agency (www.emea.europa.eu/).
We planned to describe any data obtained from these sources.
Data collection and analysis
We used EndNote X4 software to manage electronic search records. We compiled handsearches using a Microsoft Excel spreadsheet and Microsoft Word documents. We created a data extraction form in a Microsoft Excel spreadsheet for each included trial. We included 'Risk of bias' assessment summaries in the Microsoft Excel spreadsheet or as a Microsoft Word document where appropriate.
Selection of studies
Two review authors (AK and RS) independently reviewed the records obtained from the various searches. We reviewed titles and abstracts and, if more information was required to determine whether the trial met the inclusion criteria, we obtained the full text. We translated articles in languages other than English. We recorded reasons for excluding studies and resolved disagreements by consensus or by discussion with a third review author (CB) if needed.
Data extraction and management
Two review authors (AK and RS) independently extracted the relevant data from included trials and entered data into Review Manager 5 using the double‐entry system (RevMan 2011). We extracted raw data (means and standard deviations (SD) for continuous outcomes and number of events or participants for dichotomous outcomes) for outcomes of interest. We extracted data from figures if not provided as raw data and calculated SDs from confidence interval (CI) estimates. If available, we extracted intention‐to‐treat analyses. When necessary, we contacted the authors of the primary studies to request additional data.
We extracted the following data:
general study information (authors, year, journal, title, study setting);
study design (study design, gout definition, inclusion and exclusion criteria, medications and comparators, dose of medications, co‐administered medications, duration of treatment and cross‐over design if applicable);
study characteristics (number of participants, range and age of participants, percentage female participants, mean duration of gout symptoms, previous gout therapies, per cent and number of participants with tophaceous gout);
study results (see Types of outcome measures including benefit and safety outcomes);
intention‐to‐treat results (outcome measures at the end of the placebo phase, and any summary measures with SDs, CIs and P values where given, drop‐out rate and reasons for withdrawal);
risk of bias measures.
Assessment of risk of bias in included studies
We assessed the potential for bias in included RCTs and CCTs using a 'Risk of bias' table. Two review authors (AK and RS) independently assessed risk of bias for all included studies for the following items: random sequence generation; allocation concealment; blinding of participants, care provider and outcome assessor for each outcome measure; incomplete outcome data and other biases (including carry‐over effect from previous therapies, whether appropriate co‐intervention (e.g. colchicine or NSAIDs) were administered and whether any pre‐administered interventions could diminish the effect of the subsequent randomised intervention), conforming to the methods recommended by The Cochrane Collaboration (Higgins 2011a).
To determine the risk of bias of a study, for each criterion we evaluated the presence of sufficient information and the likelihood of potential bias. We rated each criterion as 'yes' (low risk of bias), 'no' (high risk of bias) or 'unclear' (either lack of information or uncertainty over the potential for bias). In a consensus meeting, we discussed and resolved disagreements among the review authors. A third review author (RB) was available to make the final decision if we did not reach consensus.
Measures of treatment effect
We planned to summarise data in a meta‐analysis only if there was sufficient clinical homogeneity. For continuous data, we analysed results as mean differences (MD) between the intervention and comparator group, with corresponding 95% CIs. The MD between treated group and control group was weighted by the inverse of the variance in the pooled treatment estimate. For dichotomous data, we calculated a risk ratio (RR) with corresponding 95% CIs. Where there had been dichotomous data from cross‐over trials combined with data from parallel‐group trials using the generic inverse variance method, we would have calculated the odds ratio (OR) with 95% CIs rather than the RR.
We planned that for studies containing more than two intervention groups, making multiple pairwise comparisons between all possible pairs of intervention groups possible, data from the same group of participants would be used only once in the meta‐analysis.
Unit of analysis issues
We did not expect unit of analysis problems in this review. In the event that we had identified cross‐over trials in which the reporting of continuous outcome data precluded paired analysis, we would not have included these data in the meta‐analysis, in order to avoid unit‐of‐analysis error. Where carry‐over effects were thought to exist, and where sufficient data existed, we included only data from the first period in the analysis (Higgins 2011b).
Dealing with missing data
Where data was missing or incomplete, we planned to seek further information from the study authors.
In cases where individuals were missing from the reported results and no further information was forthcoming from the study authors, we planned to assume the missing values to have a poor outcome.
For dichotomous outcomes that measured AEs (e.g. number of withdrawals due to AEs), we calculated the withdrawal rate using the number of people that received treatment as the denominator (worst‐case analysis). For dichotomous outcomes that measured benefits, we calculated the worst‐case analysis using the number of randomised participants as the denominator.
For continuous outcomes (e.g. pain), we calculated the MD or standardised mean difference (SMD) based on the number of people analysed at the time point. When the number of people analysed was not presented for each time point, we used the number of randomised people in each group at baseline.
Where possible, we computed missing SDs from other statistics such as standard errors, CIs or P values, according to the methods recommended in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). If we could not calculate SDs, we imputed them (e.g. from other studies in the meta‐analysis) (Higgins 2011b).
Assessment of heterogeneity
Prior to meta‐analysis, we assessed studies for clinical homogeneity with respect to type of therapy, control group and the outcomes. For any studies judged as clinically homogeneous, we planned to assess statistical heterogeneity using the I2 statistic (Deeks 2011), using the following as an approximate guide for interpretation:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity.
In cases of considerable heterogeneity (defined as I2 ≥ 75%), we planned to explore the data further, including subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
In order to determine whether reporting bias was present, we determined whether the protocol of the RCT was published before recruitment of participants of the study was started. For studies published after 1 July 2005, we screened the Clinical Trial Register at the International Clinical Trials Registry Platform of the WHO (apps.who.int/trialssearch; DeAngelis 2004). We also evaluated whether selective reporting of outcomes was present (outcome reporting bias).
We planned to compare the fixed‐effect estimate against the random‐effects model to assess the possible presence of small‐sample bias in the published literature (i.e. in which the intervention effect was more beneficial in smaller studies). In the presence of small‐sample bias, the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate (Sterne 2011).
We would have explored the potential for reporting bias further using funnel plots if we had found 10 studies or more.
Data synthesis
Where studies were sufficiently homogeneous that it remained clinically meaningful for them to be pooled, we performed meta‐analysis using a random‐effects model, regardless of the I2 statistic results. We performed analyses using Review Manager 5 (RevMan 2011), and produced forest plots for all analyses.
Presentation of key results
We presented the main outcomes of the review in 'Summary of findings' tables. These tables provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on our seven main outcomes (participant‐reported reduction in acute gout attack frequency, per cent of participants achieving a normal serum urate level, joint pain reduction, function, participant global assessment of treatment success, withdrawal due to AEs and total AEs), as recommended by The Cochrane Collaboration (Schünemann 2011a). It includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011b). We decided post‐hoc that the main comparison (presented in Table 1) was the uricosuric medication, benzbromarone compared with a commonly used treatment, allopurinol.
In addition to the absolute and relative magnitude of effect provided in the 'Summary of findings' tables, for dichotomous outcomes, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) where appropriate from the control group event rate (unless the population event rate was known) and the RR using the Visual Rx NNT calculator (Cates 2008).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses to examine the effect of treatment on the primary benefit outcome, acute gout attacks:
abnormal renal function versus normal renal function: uricosuric agents are dependent on glomerular filtration to enter the proximal tubule and thus people with abnormal renal function may differ in their response to comparable doses of uricosurics;
different baseline serum urate levels: for example less than 6 mg/dL (less than 360 µmol/L) versus 6 to less than 9 mg/dL (360 to less than 540 µmol/L) versus 9 mg/dL or greater (540 µmol/L or greater).
Ideally, we would have extracted the outcome 'proportion of people with acute gout attacks' separately for the subgroup of the trial's population with abnormal renal function and for people with normal renal function from within each trial; and extracted the proportion of people with acute gout attacks for the subgroups of the population with low and high serum urate levels from within each trial. We planned to compare informally the magnitudes of effect to assess possible differences in response to treatment by considering the overlap of the CIs of the summary estimates in the two subgroups ‐ non‐overlap of the CIs indicating statistical significance. However, we anticipated that the outcomes may not be reported by subgroups within the trials, precluding the planned analyses.
Sensitivity analysis
Where sufficient studies existed, we planned sensitivity analyses to assess the impact of any bias attributable to inadequate or unclear treatment allocation (including studies with quasi‐randomised designs) or lack of blinding.
Results
Description of studies
Results of the search
We first ran an electronic search on 23 June 2012 and updated it on 13 May 2013. We retrieved 2099 references (see Figure 1). After excluding 407 duplicates references, we screened 1692 references, of which, 107 full‐text articles were assessed for eligibility. Five trials fulfilled the inclusion criteria (Liang 1994; Perez‐Ruiz 1999; Reinders 2009a; Reinders 2009b; Scott 1966a).
1.
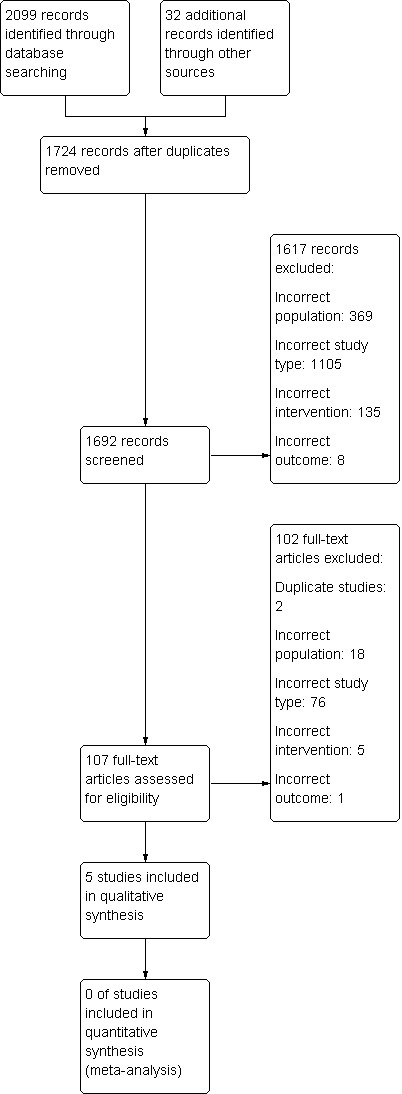
Study flow diagram.
Included studies
Design
Four RCTs, involving 237 participants, met our inclusion criteria (Liang 1994; Perez‐Ruiz 1999; Reinders 2009a; Reinders 2009b). One CCT of 37 participants also met our inclusion criteria (Scott 1966a). See Characteristics of included studies table.
Participants
Reinders 2009a and Reinders 2009b were both performed in the Netherlands and used the following definition for chronic gout: a diagnosis of gout (with either confirmation of synovial/peri‐articular urate crystals or presence of tophi) and an indication for serum urate‐lowering therapy such as the presence of tophi or frequent acute gout attacks (two/year). Liang 1994, a study completed in China, included people with a diagnosis of gout (1977 American Rheumatism Association (ARA) criteria for gout (Wallace 1977), and Holmes criteria (Holmes 1985)). Perez‐Ruiz 1999, a study completed in Spain, also utilised the ARA preliminary criteria for gout (Wallace 1977). Scott 1966a, a UK‐based RCT, defined their study population as having gout that was "as far as could be determined, primary and uncomplicated except in some cases with minor degrees of renal functional impairment".
Scott 1966a specified that the included participants must be over 18 years of age while the other studies did not specify a minimum age (Liang 1994; Perez‐Ruiz 1999; Reinders 2009a; Reinders 2009b). Scott 1966a included only men whereas all of the other studies had a majority of males (Liang 1994; Perez‐Ruiz 1999; Reinders 2009a; Reinders 2009b).
Intervention and comparator
Two trials compared benzbromarone with allopurinol (Perez‐Ruiz 1999; Reinders 2009a), two trials compared benzbromarone with probenecid (Liang 1994; Reinders 2009b), and one trial compared allopurinol with probenecid (Scott 1966a). Reinders 2009b studied participants who had not responded to a standardised two months of allopurinol therapy. One study compared probenecid with allopurinol (Scott 1966a), but 5/17 (29%) participants on probenecid changed to sulphinpyrazone 400 mg daily due to 'minor' AEs.
Outcomes
All trials measured the number of acute attacks of gout, serum urate change or normalisation, withdrawal due to AEs and total AEs. Two trials assessed tophus regression (Perez‐Ruiz 1999; Scott 1966a). The included trials did not report other OMERACT proposed domains (pain, function, participant global assessment or health‐related quality of life measures).
Benzbromarone versus allopurinol
Reinders 2009a randomised 65 participants (and an additional three participants who were later found to not meet inclusion criteria) with chronic gout to receive either benzbromarone (29 participants, 83% men, mean (SD) age 59.6 (11.3) years) or allopurinol (36 participants, 81% men, mean (SD) age 58.6 (12.3) years). Participants were started on either allopurinol (weekly step‐up dosing from 100 to 300 mg daily) or benzbromarone 100 mg daily. If, after two months, a normal serum urate of 5 mg/dL or less (0.30 mmol/L or less) was not reached, the medication doses were doubled to either allopurinol 300 mg twice daily and benzbromarone 200 mg daily. Participants also received colchicine 0.5 to 1 mg daily (or NSAIDs if colchicine was not tolerated) until serum urate normalisation of 5 mg/dL or less (0.30 mmol/L or less) was reached. Follow‐up was four months. Data were extracted from the four‐month results. The primary outcome was the percentage of participants who tolerated serum urate‐lowering medication without AEs (tolerability was not fully described) and attained a serum urate of 5 mg/dL or less (0.30 mmol/L or less). Secondary outcomes were percentage change in serum urate level, incidence of acute gout attacks over the course of the respective trials, withdrawal due to AEs and the total number of AEs.
Perez‐Ruiz 1999 randomised 37 participants but did not describe data for one individual who died after three months of therapy. Of the 36 participants for whom data were presented, 31 were men (86%)). Participants were treated with benzbromarone (17 participants, mean (SD) age 60.9 (12.8) years) or allopurinol (19 participants, mean (SD) age 67.3 (9.59) years) and follow‐up was between nine and 24 months. Benzbromarone‐treated participants were started on 100 mg daily with step‐up dosing to a maximum of 200 mg daily. Allopurinol‐treated participants began at a dose of 100 mg daily and further step‐up dosing to a maximum of 300 mg daily was based on renal function. Participants also received colchicine 0.5 to 1 mg daily for the first six months of the study. Follow‐up was nine months for participants who achieved serum urate normalisation 6 mg/dL or less (0.36 mmol/L or less). Participants who did not achieve serum urate normalisation of 6 mg/dL or less (0.36 mmol/L or less) were followed for up to 24 months on the alternative therapy. Data were extracted from the first randomised stage (nine‐month data) although the report did not fully describe the exact timing of urate measurements. The primary outcome were the proportion of participants who achieved a serum urate of 6 mg/dL or less (0.36 mmol/L or less). Tophus regression presented for all study participants rather than as a treatment specific result.
Benzbromarone versus probenecid
Reinders 2009b began by treating 93 participants with allopurinol (weekly step‐up dosing from 100 to 300 mg daily) for two months. Sixty‐two participants who had not reached serum urate normalisation of 5 mg/dL or less (0.30 mmol/L or less) at two months were subsequently randomised to step‐up therapy with either benzbromarone, target dose of 200 mg daily (27 participants, 100% men, mean (SD) age 55 (16) years) or probenecid, target dose of 1000 mg twice daily (35 participants, 94% men, mean (SD) age 58 (12) years). Participants also received colchicine 0.5 to 1 mg daily (or NSAIDs if colchicine was not tolerated) until serum urate normalisation 5 mg/dL or less was reached. Follow‐up for the randomised phase was two months and data were extracted at this time point. The primary outcome was the percentage of participants who tolerated serum urate‐lowering medication without AEs (tolerability was not fully described) and attained a serum urate of 5 mg/dL or less. Secondary outcomes were percentage change in serum urate level, incidence of acute gout attacks over the course of the respective trials, withdrawal due to AEs and the total number of AEs.
Liang 1994 randomised 74 participants to benzbromarone 50 mg daily (39 participants, 97% men, mean (SD) age 53 (13) years) or probenecid (starting dose 500 mg daily increasing over 10 days to 500 mg three times daily) (35 participants, 97% men, mean (SD) age 51 (12) years). Participants were followed for 12 weeks with data collected at one, four and 12 weeks. For the purposes of this analysis, data were extracted from the 12‐week data. The outcome assessed included percentage change in serum urate, incidence of acute gout attacks, withdrawal due to AEs and the total number of participants experiencing AEs.
Probenecid versus allopurinol
Scott 1966a performed a single‐centre open quasi‐randomised CCT including 40 participants with gout (investigator defined) comparing allopurinol versus a uricosuric (probenecid initially, then 5/17 participants on probenecid changed to sulphinpyrazone 400 mg daily due to 'minor' AEs). Allopurinol was commenced at 300 mg daily and increased when necessary (study authors have not defined how) up to 600 mg daily, and probenecid 1 g daily rising to 2 g daily after two weeks. All participants also received colchicine 0.5 mg twice or three times daily and this was withdrawn "several months after the last attack of gout". The mean follow‐up was 18.6 months for allopurinol and 19.6 months for probenecid, and outcomes were assessed at initial assessment; two weeks; one, two and three months; and at three‐monthly intervals thereafter. Outcome assessments were made on three out of the seven essential domains proposed by OMERACT. The study end‐points were acute gout attack frequency, serum urate and tophus regression. Safety as assessed by the number of study participant withdrawals due to AEs were also reported, although SAEs were not reported.
Excluded studies
The Characteristics of excluded studies table details the reasons for exclusion of the 103 excluded studies. In summary, the reasons for exclusion were as follows: two were duplicate studies, 18 were of the wrong population (e.g. not chronic gout), 76 were of the wrong study type, five were not studies of uricosuric medications and one did not include the predefined outcomes of interest.
Risk of bias in included studies
Summary assessment of the risk of bias of included studies is presented in the Characteristics of included studies table and Figure 2 and Figure 3. No studies were completely free from bias.
2.
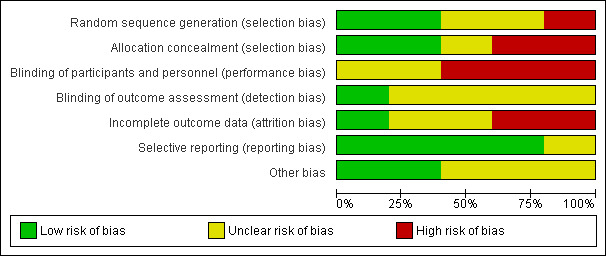
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
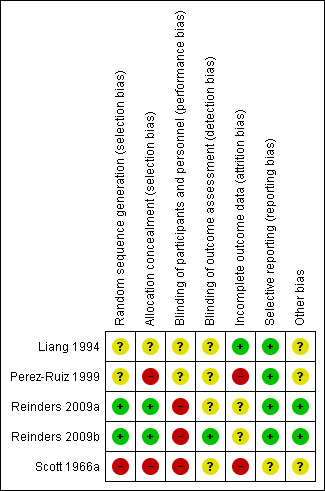
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both Reinders et al. studies utilised a central computer‐generated randomisation schedule in block sizes of six to randomise participants (Reinders 2009a; Reinders 2009b). Liang 1994 utilised a randomisation table also resulting in a low risk of bias. Perez‐Ruiz 1999 also used randomisation but no further details were provided resulting in an unclear risk of bias. Scott 1966a used a method based on the participant's hospital number leading to a high risk of bias with respect to allocation. None of the studies discussed treatment allocation concealment.
Blinding
All trials except Liang 1994 commented that their study design was open‐labelled. As these trials were open‐labelled, a performance bias is possible for all outcomes resulting in a high risk of bias. It is unlikely that there would be a detection bias regarding the outcome of serum urate normalisation but a detection bias is possible for the other outcomes including AEs, withdrawal due to AEs and acute gout attacks (unclear risk of bias). Liang 1994 had insufficient information regarding blinding and was deemed an unclear risk of bias.
Incomplete outcome data
There is an unclear risk of bias with respect to attrition given the number of participants who withdrew from Reinders 2009a and Reinders 2009b. In Reinders 2009a, 15/65 (23%) participants withdrew from the study. Similarly, in Reinders 2009b 7/62 participants (11%) randomised in the second stage to either benzbromarone or probenecid withdrew from the study. The number of withdrawals were higher for allopurinol (10 participants) than for benzbromarone (five participants) (not statistically significant) in Reinders 2009a but were for similar reasons when comparing the two groups. In Reinders 2009b, similar numbers of participants withdrew from the benzbromarone (three participants) and probenecid (four participants) groups with similar reasons for withdrawal reported.
In Reinders 2009a, 10 participants were excluded from analysis from allopurinol (six lost to follow‐up, three due to protocol violation and one not mentioned in results), while five participants were excluded from analysis from benzbromarone (four loss to follow‐up and one poor adherence). In the uricosuric treatment stage of Reinders 2009b, three participants were excluded from the benzbromarone study arm (two lost to follow‐up and one protocol violation), while four participants were excluded from the probenecid study arm (one poor compliance, two lost to follow‐up and one protocol violation).
In Perez‐Ruiz 1999, one participant had his data censored after he died three months into therapy. The therapy this participant received was not included. There was no further details of attrition bias but, as there was varying durations of follow‐up, it is possible that this could have contributed to bias and, therefore, we deemed it to have a high risk of bias.
Liang 1994 indicated in their results that all participant data were collected even though three participants stopped medication due to serious gastrointestinal issues (low risk of bias).
In Scott 1966a, data were censored from three participants (one from allopurinol and two from probenecid) who were lost to follow‐up but no further details were provided. As the results were thus incomplete, we judged this study to be a high risk of bias.
Selective reporting
There was no selective reporting apparent in all except one of the included trials. The Reinders 2009b trial was registered in the Clinical Trials Registry Platform of the WHO (NTR901), and indicated the same primary and secondary outcomes as listed in the final study report. Reinders 2009a was registered in the Netherlands Trial Register (NTR903), with the same primary and secondary outcomes. It was unclear if Scott 1966a was at risk of selective reporting.
Other potential sources of bias
Other potential sources of bias were assessed including whether there was carry‐over effect from previous therapies, whether appropriate co‐interventions (e.g. colchicine or NSAIDs) were administered and whether any pre‐administered interventions could diminish the effect of the subsequent randomised intervention. We identified no other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Pooling of results was limited due to differences in comparators. We could perform no sensitivity or subgroup analyses due to lack of data.
Benzbromarone versus allopurinol
In Reinders 2009a, there was no significant difference in the frequency of acute gout attacks observed between participants treated with benzbromarone or allopurinol after four months (1/25 with benzbromarone versus 0/30 with allopurinol; RR 3.58, 95% CI 0.15 to 84.13) (Analysis 1.1). Utilising pooled results from the two studies of benzbromarone and allopurinol (Reinders 2009a: target 5 mg/dL or less (0.3 mmol/L or less), four months; Perez‐Ruiz 1999: target 6 mg/dL or less, nine months), there was no significant difference with respect to the per cent of participants achieving serum urate normalisation using the study's respective target serum urate (34/46 with benzbromarone versus 33/55 with allopurinol; pooled RR 1.27, 95% CI 0.90 to 1.79) (Analysis 1.2). None of our other pre‐specified benefit outcomes were reported.
1.1. Analysis.
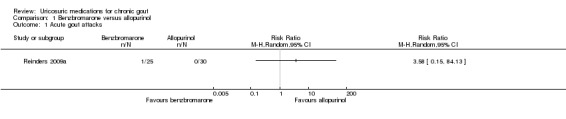
Comparison 1 Benzbromarone versus allopurinol, Outcome 1 Acute gout attacks.
1.2. Analysis.
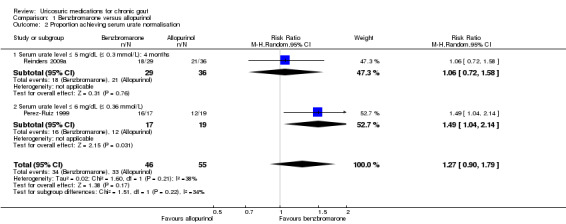
Comparison 1 Benzbromarone versus allopurinol, Outcome 2 Proportion achieving serum urate normalisation.
There was no significant difference in withdrawal due to AEs in pooled results between participants treated with benzbromarone or allopurinol (3/42 with benzbromarone versus 3/49 with allopurinol; pooled RR 1.25, 95% CI 0.28 to 5.62) (Analysis 1.3). Three participants in the benzbromarone group withdrew, one with dizziness and flushing and two with gastrointestinal reactions while three participants in the allopurinol group withdrew due to skin rashes. One additional benzbromarone‐treated participant was temporarily taken off treatment when he developed diarrhoea but he was not included in this analysis as benzbromarone was thereafter successfully restarted and the AE was determined to be a result of colchicine (Perez‐Ruiz 1999). There were no between‐group differences in Reinders 2009a in the total number of participants experiencing AEs (5/25 with benzbromarone versus 2/30 with allopurinol; RR 3.00, 95% CI 0.64 to 14.16) (Analysis 1.4). Five participants experienced AEs in the benzbromarone group (two with gastrointestinal symptoms, one with an acute gout attack, one with dizziness and flushing, and one who had an increase in international normalization ratio (INR)) and two experienced AEs in the allopurinol group (two rash/skin reactions).
1.3. Analysis.
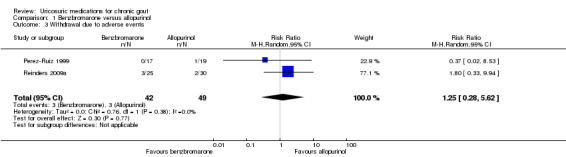
Comparison 1 Benzbromarone versus allopurinol, Outcome 3 Withdrawal due to adverse events.
1.4. Analysis.
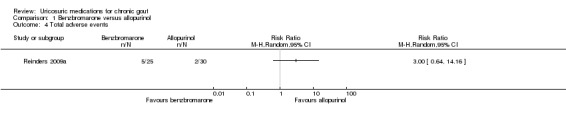
Comparison 1 Benzbromarone versus allopurinol, Outcome 4 Total adverse events.
Benzbromarone versus probenecid
The frequency of acute gout attacks was significantly different in the two trials comparing benzbromarone with probenecid (4/63 with benzbromarone versus 7/66 with probenecid; pooled RR 0.73, 95% CI 0.09 to 5.83) (Analysis 2.1). Reinders 2009b found that participants receiving benzbromarone were significantly more likely than participants receiving probenecid to achieve serum urate normalisation of 5 mg/dL or less after two months (22/27 with benzbromarone versus 20/35 with probenecid; RR 1.43, 95% CI 1.02 to 2.00) (Analysis 2.2). After 12 weeks of therapy with either benzbromarone or probenecid, Liang 1994 found no significant difference in the absolute serum urate reductions (MD 53.24 mmol/L, 95% CI ‐11.98 to 118.46) (Analysis 2.3). None of our other pre‐specified benefit outcomes were reported.
2.1. Analysis.
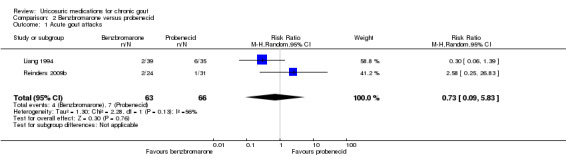
Comparison 2 Benzbromarone versus probenecid, Outcome 1 Acute gout attacks.
2.2. Analysis.
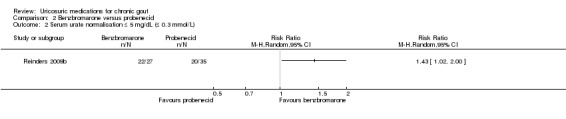
Comparison 2 Benzbromarone versus probenecid, Outcome 2 Serum urate normalisation ≤ 5 mg/dL (≤ 0.3 mmol/L).
2.3. Analysis.
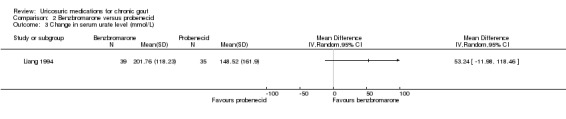
Comparison 2 Benzbromarone versus probenecid, Outcome 3 Change in serum urate level (mmol/L).
There were significantly fewer withdrawals due to AEs in participants treated with benzbromarone compared with participants treated with probenecid (1/63 with benzbromarone versus 11/66 with probenecid; pooled RR 0.15, 95% CI 0.03 to 0.79) (Analysis 2.4). One participant withdrew from the benzbromarone group following an acute gout flare while 11 participants withdrew from the probenecid group for reasons including dizziness, fatigue, gastrointestinal AEs, headache, rash and flushing. There was also significantly fewer participants experiencing an AE when treated with benzbromarone compared with probenecid (13/63 with benzbromarone versus 31/66 with probenecid; pooled RR ‐0.27, 95% CI ‐0.42 to ‐0.11) (Analysis 2.5). For the benzbromarone‐treated participants, the types of AEs reported included gastrointestinal AEs, acute gout flares, rashes and reduced leukocytes. For probenecid‐treated participants, the types of AEs reported included dizziness, fatigue, gastrointestinal AEs, acute gout flares, reduced sodium, reduced leukocytes, headache, rash and flushing.
2.4. Analysis.
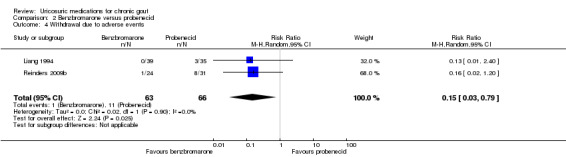
Comparison 2 Benzbromarone versus probenecid, Outcome 4 Withdrawal due to adverse events.
2.5. Analysis.
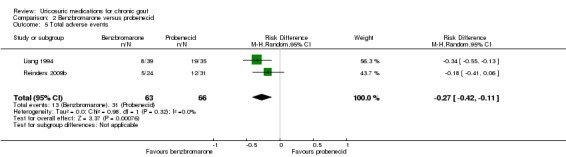
Comparison 2 Benzbromarone versus probenecid, Outcome 5 Total adverse events.
Probenecid versus allopurinol
The Scott 1966a trial compared probenecid (target dose 2 g daily) with allopurinol (300 to 600 mg daily). From the data presented, there was no difference in the number of participants who experienced an acute gout attack while on therapy with either probenecid or allopurinol (9/17 with probenecid versus 11/20 with allopurinol; RR 0.96, 95% CI 0.53 to 1.75) (analysis performed by the review authors). Mean (range) serum urate was reported to decrease from 8.5 mg/dL (7.5 to 11.7) at baseline to 5.2 mg/dL (3.8 to 7.3) at final endpoint in the probenecid group and 9.3 mg/dL (7.5 to 10.6) at baseline to 4.7 mg/dL (2.6 to 5.5) at the final endpoint in the allopurinol group (no measures of variance were reported). Of people with tophi (five overall), disappearance of tophi occurred in one of two in the probenecid group and two of three participants in the allopurinol group. None of our other pre‐specified benefit outcomes were reported. There did not appear to be any between‐group difference in number of AEs and no SAEs were reported in either group. Of note, 29% (5/17) of probenecid‐treated participants changed to sulphinpyrazone due to what were described as minor AEs.
Further safety assessment of the uricosurics
A search of the European Public Assessment Reports and Australian Database of Adverse Event Notifications found no reports of SAEs of uricosurics, but did note the potential for drug interactions between uricosurics (e.g. probenecid) and aspirin, ibuprofen and paracetamol.
Reports from a search of MedWatch, which includes FDA AE reporting, discussed how probenecid interferes with the renal tubular transfer of cefotaxime leading to an approximate 50% decrease in total clearance of cefotaxime (FDA Center for Drug Evaluation and Research (CDER) ‐ September 2011).
From the UK Medicine and Health Care products Regulatory agency, there were no Drug Safety Updates on any of the uricosuric medications. Drug analysis prints reported 12 reactions to benzbromarone of which one was jaundice and a second was elevated transaminases. Probenecid had 89 reported reactions, of which four were fatal (thrombocytopenia, sudden death, leukaemia and encephalopathy). Sulphinpyrazone had 112 reported reactions, of which four were fatal (aplastic anaemia, cardiac arrest, sepsis and Stevens‐Johnson syndrome).
Discussion
This systematic review analysed the evidence from all published RCTs and CCTs of uricosuric medications in the treatment of chronic gout. We included four RCTs and one CCT. Two studies assessed benzbromarone versus allopurinol therapy: Reinders 2009a (65 participants for four months) and Perez‐Ruiz 1999 (36 participants for nine to 24 months). Two studies also assessed benzbromarone versus probenecid: Reinders 2009b (62 participants for two months) and Liang 1994 (74 participants for 12 weeks). One CCT assessed probenecid versus allopurinol (37 participants for a mean of 19.6 months with probenecid or 18.6 months with allopurinol; Scott 1966a).
Summary of main results
There was low‐quality evidence based on one small trial (65 participants) that there is no significant difference in acute gout attacks or the total number of AEs when comparing benzbromarone (up to 200 mg daily) and allopurinol (up to 300 mg twice daily). There was also no significant difference in the number of participants who achieved serum urate normalisation (moderate‐quality evidence) or the number of participants who withdrew due to AEs (low‐quality evidence) in two trials (102 participants) comparing benzbromarone with allopurinol (Table 1). Due to the small number of participants in these trials, there is a risk that these studies could not detect a significant difference in outcomes.
There was low‐quality evidence based on two small trials (136 participants) that benzbromarone (50‐200 mg daily) is no more likely than probenecid (1500 to 2000 mg total daily in divided doses) to result in acute gout attacks. While one study indicated that benzbromarone was 1.4 times more likely to achieve serum urate normalisation of 5 mg/dL or less (0.3 mmol/L or less) than probenecid in people with chronic gout (moderate‐quality evidence; 82% with benzbromarone versus 57% with probenecid) with an NNTB of five people (Reinders 2009b), the second study found no significant difference in the total reduction in serum urate when comparing benzbromarone with probenecid (Liang 1994) (Table 2). Based upon the evidence of these two trials, benzbromarone was 6.7 times less likely to result in withdrawals due to AEs (2% with benzbromarone versus 17% with probenecid) with an NNTB of seven people. Benzbromarone was also 2.3 times less likely to result in a participant experiencing an AE (21% with benzbromarone versus 47% with probenecid) with an NNTB of four people.
There was low‐quality evidence based on one small CCT (37 participants) that there was no significant difference between probenecid (2 g daily) and allopurinol (300 to 600 mg daily) in acute gout attacks (Table 3).
There was insufficient evidence to draw conclusions on how uricosurics compare with other urate‐lowering therapies such as xanthine oxidase inhibitors (allopurinol and febuxostat). However, as reviewed in Seth 2013 and Tayar 2012, there is a larger overall body of higher‐quality evidence examining allopurinol and febuxostat in people with gout (although not in comparison to uricosurics). Given the relative dearth of studies examining the benefit and safety of uricosurics, it is reasonable to consider uricosurics as second‐line agents in the treatment of gout.
Overall completeness and applicability of evidence
We have included the only four published RCTs and one CCT examining the benefit and safety of uricosuric medications in the treatment of chronic gout. Two RCTs examined a benzbromarone versus allopurinol (Perez‐Ruiz 1999; Reinders 2009a), while two RCTs examined benzbromarone versus probenecid (Liang 1994; Reinders 2009b). One CCT examined the benefit of probenecid versus allopurinol (Scott 1966a). Based on the inclusion criteria of these trials, the results are most relevant to males aged in their 50s to 60s without significant renal or liver disease.
As benzbromarone is not currently available in many countries, there is also a limitation to the applicability of these data to current practice. We identified no RCTs of sulphinpyrazone.
Quality of the evidence
The body of evidence for uricosuric medications was limited by a paucity of RCTs. We identified only five trials, two for benzbromarone versus allopurinol, two for benzbromarone versus probenecid and one for probenecid versus allopurinol. The trials were limited by their small size (Reinders 2009a: 65 participants; Perez‐Ruiz 1999: 36 participants; Reinders 2009b: 63 participants; Liang 1994: 74 participants; Scott 1966a: 37 participants). In addition, the studies by the Reinders group (Reinders 2009a; Reinders 2009b), and the study by Liang 1994 were limited by their short duration (four months (Reinders 2009a), two months (Reinders 2009b), and 12 weeks (Liang 1994)). Liang 1994 also utilised a relatively low dose of benzbromarone, which may limit the ability of this trial to demonstrate a difference in benefit compared with probenecid. Both Perez‐Ruiz 1999 and Scott 1966a had limitations related to the variable duration of follow‐up. No trial included a placebo arm and all trials were open‐label rendering them at risk of performance and detection bias. As many of the trials were small, there was a risk that they lacked power to detect differences in the outcomes discussed and could have imprecision. Thus, we downgraded the evidence for the outcome, proportion achieving serum urate normalisation to moderate and the outcomes acute gout attacks, withdrawals due to AEs, and total AEs to low. No trial included an assessment of participant‐relevant outcomes such as pain, health‐related quality of life, function or participant global assessment of treatment success.
The studies of uricosuric medications were also limited by indirectness in that there were no comparisons available to newer urate‐lowering therapies such as febuxostat or pegloticase. No analyses were available for subgroups of participants such as people with renal insufficiency in whom efficacy of these medications likely differs. There did not appear to be a high probability of publication bias.
Potential biases in the review process
We are confident that the broad literature search used in this review has captured relevant literature and minimised the likelihood that we missed relevant trials. However, the time lag between search date and publication date may be a potential source of bias. Two review authors performed an independent review of all abstracts and titles as well as data extraction and risk of bias assessment. We reached consensus after discussing any discrepancies thus minimising bias. Protocols for both included studies were published with pre‐defined benefit and safety outcomes. To address more rare SAE, we also searched regulatory agency reports. We excluded one study, as it did not report data for the outcomes that were included in this review (Müller 1993b). It is likely that the study did collect such data but it was not presented as such. It is highly unlikely that the results of this pharmacokinetic study would change the results of this review as it included a small number of participants (14 total) and treatment was only for seven days on each medication.
Agreements and disagreements with other studies or reviews
Both the ACR Gout Guidelines (Khanna 2012), and another set of gout recommendations (Hamburger 2011), have systematically reviewed the literature and suggested a role for uricosuric medications, in particular probenecid, as an option in the management of gout.
Hamburger 2011 performed a review that included some of the same RCTs and CCTs that were included in our review. Their review was limited to English language studies and thus did not include the Liang 1994 study. They also did not discuss Perez‐Ruiz 1999 or Scott 1966a study. Hamburger 2011 also included data from uncontrolled trials (e.g. Reinders 2007;Stocker 2011), which we had excluded, as well as studies in asymptomatic people with hyperuricaemia (Stocker 2008). Their results differed from ours in that they only reviewed the benefit of probenecid and probenecid in combination with xanthine oxidase inhibitors as this is the available uricosuric medication in the US. A descriptive analysis was provided and no meta‐analysis was performed due to the small number of studies identified.
The ACR guidelines do not detail the included articles in their guidelines but focused on probenecid as that is currently the only available uricosuric medication in the US. In reviewing their recommendations, they also cited an observational study (Perez‐Ruiz 2002), and a non‐randomised trial (Takahashi 2003), examining benefit of uricosurics. The ACR guidelines recommend that probenecid be used as a second‐line agent in people who have not responded to a xanthine oxidase inhibitor (allopurinol or febuxostat) (Khanna 2012), and the Hamburger recommendations suggest that probenecid is an alternative to the xanthine oxidase inhibitors that are considered first line (Hamburger 2011). The results of this Cochrane review agree in that they indicate that the uricosurics (including probenecid, and benzbromarone where available) are effective at lowering uric acid levels and are an option for the management of people with chronic gout. However, the order in which to use urate‐lowering therapies is based largely on expert opinion rather than evidence from the literature.
Neither review discussed the possible AEs related to uricosuric therapy with the exception of the increased risk of urolithiasis and the need to screen for elevated urinary uric acid level prior to initiation of uricosuric treatment (Hamburger 2011; Khanna 2011). The safety data collected in our review from RCT and CCTs did not report urolithiasis as an AE. AEs reported for the uricosurics in the trials included in our review included acute gout attacks, dizziness, fatigue, gastrointestinal AEs, headache, rash, flushing, reduced sodium and reduced leukocytes. Hepatotoxicity was also not encountered in the studies of benzbromarone.
Authors' conclusions
Implications for practice.
Uricosuric agents such as benzbromarone and probenecid can act to lower serum urate levels in people with chronic gout. From the available study results, it was not possible to detect a difference in the benefit of benzbromarone and allopurinol therapy (Perez‐Ruiz 1999; Reinders 2009a). From the results of two studies, there were mixed results regarding the benefit of benzbromarone compared with probenecid when measured as either the ability to achieve serum urate normalisation or the absolute decrease in serum urate (Liang 1994; Reinders 2009b). However, benzbromarone did result in fewer withdrawals due to adverse events and fewer total participants experiencing adverse events compared with probenecid. We downgraded the evidence because of a possible risk of performance and other biases and imprecision, so there is some uncertainty around the effect estimates.
There was insufficient evidence to draw conclusions on how uricosurics compare with other urate‐lowering therapies, such as xanthine oxidase inhibitors (allopurinol and febuxostat). However, as reviewed in Seth 2013 and Tayar 2012, there is a larger overall body of higher‐quality evidence examining allopurinol and febuxostat in people with gout (although not in comparison to uricosurics). Given the relative dearth of studies examining the benefit and safety of uricosurics, it is reasonable to consider uricosurics as second‐line agents in the treatment of gout.
Implications for research.
Further randomised controlled trials (RCTs) of uricosuric medications in chronic gout would be beneficial in expanding the body of literature on the benefit and safety of these medications. These trials should consider the Outcome Measures in Rheumatology Meeting (OMERACT) recommendations for outcome measurement and include measures of pain, health‐related quality of life, function, participant global assessment of treatment success and tophus regression as well achievement of serum urate normalisation, incidence of acute gout attacks and adverse events. Clinical recommendations regarding gout therapy are currently limited by the lack of studies of uricosurics and specifically by the lack of studies comparing uricosurics with other agents directly (e.g. febuxostat) or with combination therapy (e.g. with allopurinol). There have been concerns regarding the possible hepatotoxicity of benzbromarone leading to the withdrawal of this medication from some markets but, unfortunately, there is little evidence in the literature to determine the incidence of this adverse event. As benzbromarone is not widely available, further studies to assess the incidence of adverse events are unlikely to occur but, in markets where benzbromarone remains available, post‐marketing surveillance should be pursued to further monitor for hepatotoxicity.
History
Protocol first published: Issue 4, 2013 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 14 April 2012 | Amended | Protocol Version |
| 3 October 2011 | Amended | Protocol |
Acknowledgements
The authors would like to thank Louise Falzon from Columbia University Medical Centre for her assistance and valuable comments in the search strategy development. We would also like to thank Marisa Chau for assistance with translation and analysis of the included Chinese language study and Simon Huang, Ian Tsang, Eugene Agranovich, Caroline van Durme, Francisca Sivera, Arnd Kleyer and Daniel Aletaha for assistance with translation of potentially relevant studies.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Gout explode all trees
#2 gout*:ti,ab
#3 tophus"ti,ab
#4 tophi:ti,ab
#5 tophaceous:ti,ab
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH descriptor Probenecid explode all trees
#8 proben*:ti,ab
#9 Ben?cid:ti,ab
#10 Benemid*:ti,ab
#11 Benuryl:ti,ab
#12 Blenox:ti,ab
#13 ColBenemid:ti,ab
#14 Degona:ti,ab
#15 Emicilin:ti,ab
#16 Gonocilin:ti,ab
#17 Gonol:ti,ab
#18 Gonorrels:ti,ab
#19 Polycillin‐PRB:ti,ab
#20 Probalan:ti,ab
#21 Probeci*:ti,ab
#22 Pro‐Cid:ti,ab
#23 Prototapen:ti,ab
#24 Solpurin:ti,ab
#25 Urocid:ti,ab
#26 Santuril:ti,ab
#27 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26)
#28 MeSH descriptor Benzbromarone, this term only
#29 benzbromar*:ti,ab
#30 Besuric:ti,ab
#31 Allobenz:ti,ab
#32 Azubromaron:ti,ab
#33 Benarone:ti,ab
#34 Comburic:ti,ab
#35 Desuric:ti,ab
#36 Desatura:ti,ab
#37 Duovitan:ti,ab
#38 Facilit:ti,ab
#39 "Gichtex plus":ti,ab
#40 Harolan:ti,ab
#41 Harpagin:ti,ab
#42 "Max Uric":ti,ab
#43 Minuric:ti,ab
#44 Obaron:ti,ab
#45 Uricovac:ti,ab
#46 Urinorm:ti,ab
#47 Urifugan:ti,ab
#48 Uroplus:ti,ab
#49 Acifugan:ti,ab
#50 Narcaricin*:ti,ab
#51 allomaron:ti,ab
#52 (#28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR#51)
#53 MeSH descriptor Sulfinpyrazone, this term only
#54 sulfin*:ti,ab
#55 sulfoxyphenylpyrazolidin:ti,ab
#56 Anturan*:ti,ab
#57 Enturen:ti,ab
#58 Falizal:ti,ab
#59 Novo‐Pyrazone:ti,ab
#60 spz:ti,ab
#61 (#53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60)
#62 (#27 OR #52 OR #61)
#63 (#6 AND #62)
Appendix 2. MEDLINE search strategy
1. exp Gout/
2. gout$.tw.
3. tophus.tw.
4. tophi.tw.
5. tophaceous.tw.
6. or/1‐5
7. Probenecid/
8. proben$.tw.
9. Ben?cid.tw.
10. Benemid$.tw.
11. Benuryl.tw.
12. Blenox.tw.
13. ColBenemid.tw.
14. Degona.tw.
15. Emicilin.tw.
16. Gonocilin.tw.
17. Gonol.tw.
18. Gonorrels.tw.
19. Polycillin‐PRB.tw.
20. Probalan.tw.
21. Probeci$.tw.
22. Pro‐Cid.tw.
23. Prototapen.tw.
24. Solpurin.tw.
25. Urocid.tw.
26. Santuril.tw.
27. or/7‐26
28. Benzbromarone/
29. benzbromar$.tw.
30. Besuric.tw.
31. Allobenz.tw.
32. Azubromaron.tw.
33. Benarone.tw.
34. Comburic.tw.
35. Desuric.tw.
36. Desatura.tw.
37. Duovitan.tw.
38. Facilit.tw.
39. Gichtex plus.tw.
40. Harolan.tw.
41. Harpagin.tw.
42. Max Uric.tw.
43. Minuric.tw.
44. Obaron.tw.
45. Uricovac.tw.
46. Urinorm.tw.
47. Urifugan.tw.
48. Uroplus.tw.
49. Acifugan.tw.
50. Narcaricin$.tw.
51. allomaron.tw.
52. or/28‐51
53. Sulfinpyrazone/
54. sulfin$.tw.
55. sulfoxyphenylpyrazolidin.tw.
56. Anturan$.tw.
57. Enturen.tw.
58. Falizal.tw.
59. Novo‐Pyrazone.tw.
60. spz.tw.
61. or/53‐59
62. or/27,52,61
63. 6 and 62
64. randomized controlled trial.pt.
65. controlled clinical trial.pt.
66. randomized.ab.
67. placebo.ab.
68. drug therapy.fs.
69. randomly.ab.
70. trial.ab.
71. groups.ab.
72. or/118‐125
73. (animals not (humans and animals)).sh.
74. 72 not 73
75. 63 and 74
76. (ae or to or po or co).fs.
77. (safe or safety).ti,ab.
78. side effect$.ti,ab.
79. ((adverse or undesirable or harms$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab.
80. exp product surveillance, postmarketing/
81. exp adverse drug reaction reporting systems/
82. exp clinical trials, phase iv/
83. exp poisoning/
84. exp substance‐related disorders/
85. exp drug toxicity/
86. exp abnormalities, drug induced/
87. exp drug monitoring/
88. exp drug hypersensitivity/
89. (toxicity or complication$ or noxious or tolerability).ti,ab.
90. exp Postoperative Complications/
91. exp Intraoperative Complications/
92. or/76‐91
93. 63 and 92
94. or/75, 93
Appendix 3. EMBASE search strategy
1. exp gout/
2. gout$.tw.
3. tophus.tw.
4. tophi.tw.
5. tophaceous.tw.
6. or/1‐5
7. uricosuric agents/
8. Probenecid/
9. proben$.tw.
10. Ben?cid.tw.
11. Benemid$.tw.
12. Benuryl.tw.
13. Blenox.tw.
14. ColBenemid.tw.
15. Degona.tw.
16. Emicilin.tw.
17. Gonocilin.tw.
18. Gonol.tw.
19. Gonorrels.tw.
20. Polycillin‐PRB.tw.
21. Probalan.tw.
22. Probeci$.tw.
23. Pro‐Cid.tw.
24. Prototapen.tw.
25. Solpurin.tw.
26. Urocid.tw.
27. Santuril.tw.
28. or/62‐82
29. benzbromarone/
30. benzbromar$.tw.
31. Besuric.tw.
32. Allobenz.tw.
33. Azubromaron.tw.
34. Benarone.tw.
35. Comburic.tw.
36. Desuric.tw.
37. Desatura.tw.
38. Duovitan.tw.
39. Facilit.tw.
40. Gichtex plus.tw.
41. Harolan.tw.
42. Harpagin.tw.
43. Max Uric.tw.
44. Minuric.tw.
45. Obaron.tw.
46. Uricovac.tw.
47. Urinorm.tw.
48. Urifugan.tw.
49. Uroplus.tw.
50. Acifugan.tw.
51. Narcaricin$.tw.
52. allomaron.tw.
53. or/84‐107
54. sulfinpyrazone/
55. sulfin$.tw.
56. sulfoxyphenylpyrazolidin.tw.
57. Anturan$.tw.
58. Enturen.tw.
59. Falizal.tw.
60. Novo‐Pyrazone.tw.
61. spz.tw.
62. or/54‐61
63. or/28,53,62
64. 6 and 63
65. (random$ or placebo$).ti,ab.
66. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
67. controlled clinical trial$.ti,ab.
68. RETRACTED ARTICLE/
69. or/120‐123
70. (animal$ not human$).sh,hw.
71. 69 not 70
72. 64 and 71
73. (ae or si or to or co).fs.
74. (safe or safety).tw.
75. side effect$.tw.
76. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).tw.
77. exp adverse drug reaction/
78. exp drug toxicity/
79. exp intoxication/
80. exp drug safety/
81. exp drug monitoring/
82. exp drug hypersensitivity/
83. exp postmarketing surveillance/
84. exp drug surveillance program/
85. exp phase iv clinical trial/
86. phase 3 clinical trial/
87. (toxicity or complication$ or noxious or tolerability).ti,ab.
88. exp postoperative complication/
89. exp Peroperative Complication/
90. or/73‐89
91. 64 and 90
92. or/72, 91
Data and analyses
Comparison 1. Benzbromarone versus allopurinol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute gout attacks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Proportion achieving serum urate normalisation | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.90, 1.79] |
| 2.1 Serum urate level ≤ 5 mg/dL (≤ 0.3 mmol/L): 4 months | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.72, 1.58] |
| 2.2 Serum urate level ≤ 6 mg/dL (≤ 0.36 mmol/L) | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.04, 2.14] |
| 3 Withdrawal due to adverse events | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.28, 5.62] |
| 4 Total adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Benzbromarone versus probenecid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute gout attacks | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.09, 5.83] |
| 2 Serum urate normalisation ≤ 5 mg/dL (≤ 0.3 mmol/L) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Change in serum urate level (mmol/L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Withdrawal due to adverse events | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.79] |
| 5 Total adverse events | 2 | 129 | Risk Difference (M‐H, Random, 95% CI) | ‐0.27 [‐0.42, ‐0.11] |
Comparison 3. Probenecid versus allopurinol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute gout attacks | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected |
3.1. Analysis.
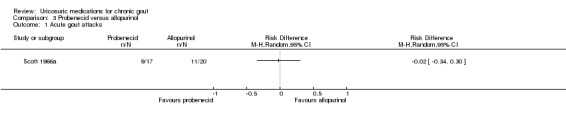
Comparison 3 Probenecid versus allopurinol, Outcome 1 Acute gout attacks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Liang 1994.
| Methods | Randomised controlled trial Follow‐up: 12 weeks No. participants randomised: 74 No. participants analysed: 74 (39 benzbromarone; 35 probenecid) |
|
| Participants | Inclusion criteria: participants with a diagnosis of gout (1977 ARA criteria for gout (Wallace 1977), and Holmes criteria (Holmes 1985)) who had not used medication that lowered blood uric acid in the half month prior to the study Exclusion criteria: history of drug allergy, liver dysfunction, moderate to severe renal dysfunction, poor compliance Location: China Baseline demographics: Mean age (SD): 52.62 year (12.53) benzbromarone; 50.71 years (11.64) probenecid Sex: 97% men in each group Tophaceous gout: 18% benzbromarone; 17% probenecid |
|
| Interventions | Benzbromarone 50 mg daily; 39 participants Probenecid: target dose 500 mg 3 times daily (titrated over 10 days from starting dose of 500 mg daily); 35 participants Concurrent therapies: all participants received baking soda, water intake > 1500 mg daily, controlled high purine diet |
|
| Outcomes | Absolute and per cent change in serum urate, urinary uric acid excretion, acute gout attacks, total adverse events Clinical efficacy scale:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "According to a randomisation table…" (translated) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: although 3 participants stopped medication due to serious gastrointestinal issues, reporting in the results section 'Clinical effectiveness' paragraph and Table 4 indicates that all participants data were collected |
| Selective reporting (reporting bias) | Low risk | All planned outcomes as defined were reported in the result |
| Other bias | Unclear risk | Unclear |
Perez‐Ruiz 1999.
| Methods | Randomised controlled trial: open and actively controlled No. participants enrolled: 37 but 1 died at 3 months No. participants analysed: 36 (17 benzbromarone; 19 allopurinol) |
|
| Participants | Inclusion criteria: all had gout as per ARA preliminary criteria; persistent CrCl from 20‐80 mL/minute, acceptance of urate‐lowering therapy Exclusion criteria: none described Location: gout clinic in a Rheumatology division, Spain |
|
| Interventions | Benzbromarone 100 mg once daily (titrated up to 200 mg once daily) Allopurinol 100‐150 mg dose starting (depending on CrCl: 100 mg daily for 20‐40 mL/minute, 200 mg daily for 40‐60 mL/minute, 300 mg daily for 60‐80 mL/minute) titrated to maximum for CrCl. Participants could cross‐over to the other group if they did not achieve serum urate level at maximum dose) Concurrent therapies: colchicine 0.5 mg once daily (if CrCl < 50 mL/minute) to 1 mg once daily (if CrCl 50‐80 mL/minute) for 6 months from the start of the study. For acute attacks, colchicine 1.5‐3 mg daily (divided into 3 doses). Duration of treatment: 9‐12 months if "proper control of serum urate" and 12‐24 months for "patients who changed from allopurinol to benzbromarone or patients with tophi" |
|
| Outcomes | Primary outcomes: acute gout attack frequency, serum urate (both change in serum urate and serum urate < 6 mg/dL) and tophus regression Secondary outcomes: adverse effects |
|
| Notes | Outcome assessments were made on 3 out of the 7 essential domains proposed by OMERACT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial: open and actively controlled |
| Allocation concealment (selection bias) | High risk | Randomised controlled trial: open and actively controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Randomised controlled trial: open and actively controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study outcomes may be influenced by lack of blinding in this case |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: "One patient died of cardiac failure 3 months after entering study, so efficacy could not be evaluated and results were available for 36 patients". "One patient taking allopurinol showed a pruritic erythematous rash that was attributed to allopurinol, and this drug was withdrawn" |
| Selective reporting (reporting bias) | Low risk | Quote: "The outcome variables were the following: reduction of serum urate, the percentage of reduction from basal serum urate, and proper control of serum uric acid (Sur <6mg/dL). The number of gouty bouts during follow‐up and the reduction of the size of tophi were also recorded. The benefit of urate‐lowering drugs on Sur was evaluated when two consecutive determinations of Sur did not differ in more than 1mg/dL" |
| Other bias | Unclear risk | No comment on washout period and variable follow up times. |
Reinders 2009a.
| Methods | Multicentre open‐label, randomised controlled trial Follow‐up: 4 months No. participants randomised: 68 No. participants analysed: 65 (3 participants did not meet inclusion criteria) |
|
| Participants | Inclusion criteria: diagnosis of gout (with either confirmation of synovial/peri‐articular urate crystals or presence of tophi), CrCl ≥ 50 mL/minute, indication for serum urate‐lowering therapy (presence of tophi or frequent attacks: 2/year) Exclusion criteria: history of having used 1 of the study drugs, relevant liver disease Location: Netherlands Baseline demographics: Mean age (SD): 58.6 years (12.3) allopurinol; 59.6 years (11.3) benzbromarone Sex: 81% male allopurinol; 83% male benzbromarone Tophaceous gout: 53% allopurinol; 48% benzbromarone |
|
| Interventions | Participants randomised to 2 groups and all participants received colchicine 0.5‐1 mg daily (or NSAIDs if colchicine was not tolerated) until serum urate normalisation was reached (≤ 5 mg/dL (≤ 0.30 mmol/L)). There were 2 stages to therapy, each lasting 2 months: Stage 1: Allopurinol weekly step‐up dosing from 100 to 200 to 300 mg daily; 36 participants Benzbromarone 100 mg daily; 29 participants Stage 2: At 2 months if serum urate normalisation (≤ 5 mg/dL (≤ 0.30 mmol/L)) was not reached: Allopurinol increased to 300 mg twice daily Benzbromarone increased to 200 mg daily |
|
| Outcomes | Primary outcomes: percentage of participants who tolerated the serum urate‐lowering medication and attained serum urate (≤ 5 mg/dL (≤ 0.30 mmol/L) after stage 1 or stage 2) Secondary outcomes: percentage change in serum urate level, withdrawal due to adverse drug reaction, mean serum oxipurinol concentration (allopurinol group), acute gout attacks, total adverse drug reactions |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated central randomisation schedule with a block size of six was used" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the time of inclusion in the study, patients were assigned an inclusion number by the rheumatologist (blinded) and subsequently randomised to allopurinol or benzbromarone treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: open‐label. Regarding serum urate normalisation, a detection bias was unlikely. For the other outcomes of this study, including adverse drug reactions, withdrawal due to adverse drug reactions and acute gout attacks, a detection bias could exist |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "Two patients stopped receiving allopurinol and three stopped receiving benzbromarone because of adverse drug reactions"; Figure 1: "10 excluded from analysis from Allopurinol: 6 lost to follow‐up, 3 protocol violation"; "5 excluded from analysis from Benzbromarone; 4 loss to follow‐up, 1 poor adherence" Comment: 15 of 65 participants withdrew from the study (10 allopurinol; 5 benzbromarone). Similar reasons for withdrawal were present between the groups |
| Selective reporting (reporting bias) | Low risk | All planned outcomes as defined in the trial registrations of both trials were reported in the results |
| Other bias | Low risk | None identified |
Reinders 2009b.
| Methods | Multicentre, prospective, open‐label, randomised controlled trial, 4 months No. participants entering stage 1: 93 No. participants randomised to stage 2: 62 No. participants analysed: 93 (stage 1) allopurinol; 27 (3 participants not eligible for analysis) benzbromarone; 35 (4 not eligible for analysis) probenecid |
|
| Participants | Inclusion criteria: diagnosis of gout confirmed by microscopic evidence of urate crystals or otherwise, complying with the ARA criteria, indication for serum urate‐lowering therapy (tophi or frequent attacks (> 2/year) Exclusion criteria: history of having used 1 of the study drugs, relevant liver disease, relevant renal disease (CrCl ≤ 50 mL/minute) Location: Netherlands Baseline demographics: Stage 1 demographics for allopurinol: Mean (SD) age: 58 years (14); 92% male; 41% tophaceous gout Stage 2 demographics for benzbromarone and probenecid groups respectively: Mean age (SD): 55 years (16) benzbromarone; and 58 years (12) probenecid Sex: 100% male benzbromarone; 94% male probenecid Tophaceous gout: 30% benzbromarone; 54% probenecid |
|
| Interventions | Participants were all treated with allopurinol in stage 1 and participants who did not reach serum urate normalisation (≤ 5 mg/dL (≤ 0.30 mmol/L)) at 2 months were subsequently randomised to 1 of 2 treatment arms for stage 2 (an additional 2 months). All participants received colchicine 0.5‐1 mg daily (or NSAIDs if colchicine was not tolerated) until serum urate was normalised (≤ 5 mg/dL (≤ 0.30 mmol/L)): Stage 1: Allopurinol weekly step‐up dosing from 100 to 200 to 300 mg daily; 93 participants Stage 2: Benzbromarone step‐up 100‐200 mg daily (dose increased at 1 week), to target of 200 mg daily Probenecid step‐up 500‐1000 mg twice daily (dose increased at 1 week), to target of 1000 mg twice daily |
|
| Outcomes |
Primary outcomes: percentage of participants who tolerated the serum urate‐lowering medication and attained serum urate (≤ 5 mg/dL (≤ 0.30 mmol/L)) after stage 1 or stage 2) Secondary outcomes: percentage change in serum urate level, withdrawal due to adverse drug reactions and total adverse drug reactions, acute gout attacks, mean serum oxipurinol level (stage 1) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐ generated central randomisation schedule with a block size of six was used" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the time of inclusion in the study, patients were assigned an inclusion number by the rheumatologist (blinded), and consequently randomised to stage 2 treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: open‐label. Regarding serum urate normalisation, a detection bias was unlikely. For the other outcomes of this study, including adverse drug reactions, withdrawal due to adverse drug reactions and acute gout attacks, a detection bias could exist |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "82/93 patients (88%) were eligible for analysis at the end stage 1; five patients were lost to follow‐up and six patients were excluded because of poor adherence based on non‐measurable serum oxipurinol levels." "Stage 2: 3 excluded from Benzbromarone (2 lost to follow‐up, 1 protocol violation) and 4 excluded from Probenecid (1 poor compliance, 2 lost to follow‐up, 1 protocol violation)" Comment: 7/62 participants withdrew from stage 2 of the study. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All planned outcomes as defined in the trial registrations of both trials were reported in the results |
| Other bias | Low risk | None identified |
Scott 1966a.
| Methods | Single‐centre open quasi‐randomised controlled trial Mean follow‐up: 18.6 months allopurinol; 19.6 months probenecid No. participants included: 40 (21 allopurinol with 1 who defaulted; 19 probenecid with 2 who defaulted) No. participants analysed: 37 (20 allopurinol; 17 probenecid) |
|
| Participants | Inclusion criteria: investigator defined gout (Quote: "Gout was, as far as could be determined, primary and uncomplicated except in some cases with minor degrees of renal functional impairment") Exclusion criteria: none described Location: Outpatient clinics at Charing Cross and West London Hospitals, UK Baseline demographics: Mean age: 54 years (both groups) Sex: 100% male (both groups) Tophaceous gout: 15% allopurinol; 12% probenecid |
|
| Interventions | Participants assigned to 1 of 2 groups Allopurinol was compared with a uricosuric (probenecid initially, then 5/17 on probenecid changed to sulphinpyrazone 400mg daily due to 'minor' adverse effects). Allopurinol: commenced at 300 mg daily and increased when necessary (not defined how) up to 600 mg daily Probenecid: 1 g daily rising to 2 g daily after 2 weeks Concurrent therapies: all participants received colchicine 0.5 mg twice or 3 times daily until "several months after the last attack of gout" |
|
| Outcomes | Outcome assessments were made on 3 out of the 7 essential domains proposed by OMERACT Outcomes: acute gout attack frequency, serum urate and tophus regression Safety, as assessed by the number of study participant withdrawals due to adverse events, were also reported, although serious adverse events were not reported Outcomes were assessed at initial assessment, 2 weeks; then 1, 2 and 3 months, and at 3‐monthly intervals thereafter |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Open trial Quote: "Patients were allocated to treatment with either allopurinol or uricosuric drugs by reference to the last digit of their hospital number; those with an even digit received allopurinol and those with an odd digit uricosuric therapy" |
| Allocation concealment (selection bias) | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: open‐label. Regarding serum urate normalisation, a detection bias was unlikely. For the other outcomes of this study, including adverse drug reactions, withdrawal due to adverse drug reactions and acute gout attacks, a detection bias could exist |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One patient taking allopurinol defaulted from follow‐up. One patient taking uricosuric therapy defaulted from follow‐up and one left the district so that adequate follow‐up was impossible. The results therefore apply to the 20 patients taking allopurinol and the 17 patients taking uricosuric therapy" Comment: it is unclear when participants were lost to follow‐up. The losses are balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence |
| Other bias | Unclear risk | Unclear |
ARA: American Rheumatism Association; CrCl: creatinine clearance; No: number; NSAID: non‐steroidal anti‐inflammatory drug; OMERACT: Outcome Measures in Rheumatology Meeting; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akkasilpa 2004 | Population: not all people with gout |
| Allwright 1974 | Study type: case study |
| Anonymous 1966 | Study type: letter |
| Anonymous 1968 | Study type: review |
| Anonymous 1968a | Study type: review |
| Baiatova 1981 | Study type: not randomised or controlled study |
| Bain 1974 | Study type: therapeutic conference |
| Balkarov 1994 | Study type: no comparator group |
| Ball 1971 | Study type: review |
| Bannwarth 1988 | Study type: review |
| Barden‐Maja 1999 | Study type: review |
| Barsom 1979 | Study type: no comparator group |
| Bayliss 1979 | Study type: review |
| Becker 2008 | Intervention: wrong intervention (allopurinol vs. febuxostat) |
| Bennack 2006 | Study type: review |
| Berg 1990 | Population: hyperuricaemia |
| Bluestone 1969 | Population: healthy participants |
| Bluestone 1980 | Study type: no comparator group |
| Boger 1955 | Study type: review and case report |
| Bollet 1981 | Study type: review |
| Bosmansky 1979 | Study type: no comparator group |
| Bosmansky 1982 | Study type: abstract |
| Bresnik 1974 | Study type: no comparator group |
| Broll 1975 | Study type: no comparator group |
| Brzozowska‐Jurkowska 1980 | Study type: observational study |
| Carrasco 1970 | Study type: case series |
| Casademont 1968 | Study type: case study |
| Cheng 2005 | Intervention: wrong intervention (allopurinol alone) |
| de Gery 1974 | Study type: pharmacodynamic study |
| Delbarre 1970 | Study type: no comparator group |
| Dorn 1982 | Population: people with hyperuricaemia and gout combined |
| Emmerson 1972 | Study type: review |
| Erill 1975 | Population: healthy participants |
| Famaey 1970 | Study type: no comparator group |
| Fanelli 1975 | Study type: review |
| Ferber 1980 | Study type: no comparator group |
| Frerick 1987 | Population: people with hyperuricaemia and gout combined |
| Gibson 1974 | Intervention: no intervention |
| Gresser 1994 | Population: healthy participants |
| Grobner 1982 | Study type: review |
| Grobner 2002 | Study type: review |
| Gunther 1968 | Study type: no comparator and not all people with gout |
| Gunther 1969 | Study type: not an RCT |
| Gutierrez 1982 | Study type: review |
| Hamburger 2011 | Study type: review |
| Hanly 2009 | Study type: economic analysis |
| Hanvivadhanakul 2002 | Population: people with hyperuricaemia and gout combined |
| Healey 1977 | Study type: review |
| Heel 1977 | Study type: no comparator and group with people with hyperuricaemia |
| Hertz 1972 | Study type: case report |
| Hollander 1968 | Study type: descriptive report and review |
| Houpt 1965 | Intervention: no comparator |
| Hutchison 1971 | Population: not people with gout |
| Jain 1974 | Study type: pharmacodynamic study |
| Jansen 2004 | Study type: letter |
| Joseph‐Ridge 2006 | Study type: review |
| Kawenoki‐Minc 1987 | Study type: not an RCT |
| Kersley 1966 | Study type: observational |
| Kersley 1966a | Study type: pharmacodynamic study |
| Kersley 1966b | Study type: review |
| Kersley 1970 | Study type: observational |
| Kersley 1973 | Study type: review |
| Kim 2003 | Study type: economic analysis |
| Klein 1968 | Population: mixed study population |
| Kotzaurek 1968 | Study type: case series |
| Kuzell 1966 | Study type: no comparator group |
| Lee 2008 | Study type: review |
| Masbernard 1976 | Study type: observational |
| Matsumoto 2011 | Study type: review |
| Matzkies 1983 | Study type: review |
| Mertz 1969 | Population: mixed population |
| Mertz 1976 | Population: people with hyperuricaemia |
| Mertz 1977a | Population: people with hyperuricaemia |
| Mertz 1977b | Population: people with hyperuricaemia |
| Mertz 1983a | Population: people with hyperuricaemia |
| Mertz 1983b | Study type: review |
| Mikkelsen 1966 | Study type: observational |
| Müller 1993a | Duplicate study |
| Müller 1993b | Lack of outcome measures: numeric data not presented regarding outcome of interest |
| Nishizawa 1978 | Population: not people with gout |
| Nyfos 1967 | Study type: observational |
| Palmer 1968 | Study type: pharmacodynamic study |
| Peitzman 1979 | Population: not people with gout |
| Perez‐Ruiz 2010 | Study type: observational study |
| Podevin 1967 | Population: not people with gout |
| Rausch‐Stroomann 1965 | Study type: no comparator group |
| Reinders 2007 | Study type: no comparator group |
| Rice 1959 | Study type: review/case series |
| Scott 1966b | Duplicate study |
| Scott 1968 | Study type: case report |
| Seebach 1967 | Study type: no comparator group |
| Serre 1970 | Study type: case series |
| Sjoberg 1966 | Study type: case series |
| Sternon 1967 | Study type: case series |
| Sutaria 2006 | Study type: review |
| Weinberger 1978 | Study type: no comparator group |
| Wortmann 2005 | Intervention: not drug of interest |
| Xie 2007 | Study type: treatment of acute gout |
| Yamamoto 1997 | Study type: pharmacodynamic study |
| Yonetsugu 1971 | Study type: pharmacodynamic study |
| Zhang 2006 | Study type: guidelines and review |
| Zollner 1970 | Study type: pharmacodynamic study |
Differences between protocol and review
We specified in the review, but not the protocol, the other sources of bias that we looked for: whether there was a carry‐over effect from previous therapies, whether appropriate co‐intervention (e.g. colchicine or non‐steroidal anti‐inflammatory drugs) were administered and whether any pre‐administered interventions could diminish the effect of the subsequent randomised intervention.
We replaced the primary and secondary outcomes by a list of major outcomes (i.e. those presented in the 'Summary of findings' tables) in the review. This was done to implement GRADE and the use of summary of findings tables.
Contributions of authors
AK drafted the review.
All authors contributed to the final version.
Sources of support
Internal sources
-
University of British Columbia, Canada.
In kind support
-
University Hospital Southampton NHS Foundation Trust, UK.
In kind support
-
Department of Clinical Epidemiology, Cabrini Hospital, Australia.
In kind support
-
Department of Epidemiology and Preventive Medicine, Monash University, Australia.
In kind support
-
Institute for Work & Health, Canada.
In kind support
External sources
No sources of support supplied
Declarations of interest
None.
New
References
References to studies included in this review
Liang 1994 {published data only}
- Liang DR, Xu N, Zhang HM, Cai YN, Zhang HL. Benzbromarone and probenecid in treating gout: a randomized controlled study. West China Medical Journal 1994:405‐8.
Perez‐Ruiz 1999 {published data only}
- Perez‐Ruiz F, Calabozo M, Fernandez‐Lopez MJ, Herrero‐Beites A, Ruiz‐Lucea E, Garcia‐Erauskin G, et al. Treatment of chronic gout in patients with renal function impairment: an open, randomized, actively controlled study. Journal of Clinical Rheumatology 1999;5(2):49‐55. [DOI] [PubMed] [Google Scholar]
Reinders 2009a {published data only}
- Reinders MK, Haagsma C, Jansen TL, Roon EN, Delsing J, Laar MAFJ, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300‐600 mg/day versus benzbromarone 100‐200 mg/day in patients with gout. Annals of the Rheumatic Diseases 2009;68(6):892‐7. [DOI] [PubMed] [Google Scholar]
Reinders 2009b {published data only}
- Reinders MK, Roon EN, Jansen TL, Delsing J, Griep EN, Hoekstra M, et al. Efficacy and tolerability of urate‐lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Annals of the Rheumatic Diseases 2009;68(1):51‐6. [DOI] [PubMed] [Google Scholar]
Scott 1966a {published data only}
- Scott JT. Comparison of allopurinol and probenecid. Annals of the Rheumatic Diseases 1966;25(6 Suppl):623‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akkasilpa 2004 {published data only}
- Akkasilpa S, Osiri M, Deesomchok U, Avihingsanon Y. The efficacy of combined low dose of allopurinol and benzbromarone compared to standard dose of allopurinol in hyperuricemia. Journal of the Medical Association of Thailand 2004;87(9):1087‐91. [PubMed] [Google Scholar]
Allwright 1974 {published data only}
- Allwright GT. Letter: a clinical impression of benzbromarone (Minuric). South African Medical Journal 1974;48:2301. [PubMed] [Google Scholar]
Anonymous 1966 {published data only}
- Anonymous. Zyloprim and other drugs in the management of gout. Medical Letter on Drugs & Therapeutics 1966;8:98‐9. [PubMed] [Google Scholar]
Anonymous 1968 {published data only}
- Anonymous. Ethebenecid (Urelim). Drug & Therapeutics Bulletin 1968;6:102‐3. [PubMed] [Google Scholar]
Anonymous 1968a {published data only}
- Anonymous. Gout and the kidney. Lancet 1968;1:961‐2. [PubMed] [Google Scholar]
Baiatova 1981 {published data only}
- Baiatova KV. Effect of long‐term base therapy on the clinical picture of gout. Terapevticheskii Arkhiv 1981;53:87‐91. [PubMed] [Google Scholar]
Bain 1974 {published data only}
- Bain LS, Galloway DB, Petrie JC, Wood RA. Gout. British Medical Journal 1974;1:446‐8. [Google Scholar]
Balkarov 1994 {published data only}
- Balkarov IM. The use of Allomaron in treating hyperuricemia. Terapevticheskii Arkhiv 1994;66:40‐2. [PubMed] [Google Scholar]
Ball 1971 {published data only}
- Ball GV, Freeman D. Management of chronic and recurrent gout. Modern Treatment 1971;8:829‐39. [PubMed] [Google Scholar]
Bannwarth 1988 {published data only}
- Bannwarth B, Netter P, Pere P, Gaucher A. Antihyperuricemic drugs [in French]. Concours Medical 1988;110:957‐62. [Google Scholar]
Barden‐Maja 1999 {published data only}
- Barden‐Maja A. Gout. Internal Medicine 1999;20:40‐1. [Google Scholar]
Barsom 1979 {published data only}
- Barsom S. Allomaron in hyperuricemia and gout. Zeitschrift fur Allgemeinmedizin 1979;55:1336‐40. [PubMed] [Google Scholar]
Bayliss 1979 {published data only}
- Bayliss RI. Gout. British Medical Journal 1979;1:1695‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2008 {published data only}
- Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph‐Ridge N. Determinants of the clinical outcomes of gout during the first year of urate‐lowering therapy. Nucleosides, Nucleotides and Nucleic Acids 2008;27(6‐7):585‐91. [DOI] [PubMed] [Google Scholar]
Bennack 2006 {published data only}
- Bennack E. Hyperuricemia: prevention of gout with drugs [in German]. Pharmazeutische Zeitung 2006;151:18‐25. [Google Scholar]
Berg 1990 {published data only}
- Berg H. Effectiveness and tolerance of long‐term uricosuric treatment. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1990;45(23):719‐20. [PubMed] [Google Scholar]
Bluestone 1969 {published data only}
- Bluestone R, Kippen I, Klinenberg JR. Effect of drugs on urate binding to plasma proteins. British Medical Journal 1969;4(5683):590‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bluestone 1980 {published data only}
- Bluestone R, Klinenberg J, Lee IK. Benzbromarone as a long‐term uricosuric agent. Advances in Experimental Medicine & Biology 1980;122A:283‐6. [DOI] [PubMed] [Google Scholar]
Boger 1955 {published data only}
- Boger WP, Strickland SC. Probenecid (Benemid); its uses and side‐effects in 2,502 patients. Archives of Internal Medicine 1955;95:83‐92. [DOI] [PubMed] [Google Scholar]
Bollet 1981 {published data only}
- Bollet AJ. Diagnostic and therapeutic aids in gout and hyperuricemia. Medical Times 1981;109:23‐31. [PubMed] [Google Scholar]
Bosmansky 1979 {published data only}
- Bosmansky K, Trnavsky K. Normurat (benzbromaron) in the treatment of gout. Zeitschrift fur Rheumatologie 1979;38:342‐8. [PubMed] [Google Scholar]
Bosmansky 1982 {published data only}
- Bosmansky K, Trnavsky K. Evaluation of the therapy effects of Acidum tienilicum, benzbromaron and allopurinol in gout patients. Zeitschrift fur Rheumatologie 1982;4:165. [Google Scholar]
Bresnik 1974 {published data only}
- Bresnik W, Muller MM. Results obtained with long‐term benzbromarone therapy (author's translation). Wiener Klinische Wochenschrift 1974;86:283‐7. [PubMed] [Google Scholar]
Broll 1975 {published data only}
- Broll H, Sochor H, Tausch G, Eberl R. Long‐term therapy with benzbromarone in uric arthritis. Wiener Medizinische Wochenschrift 1975;125:546‐8. [PubMed] [Google Scholar]
Brzozowska‐Jurkowska 1980 {published data only}
- Brzozowska‐Jurkowska AM. Effect of benziodarone and benzbromarone on the uric acid level in the blood and urine and on the incidence of gout attacks. Reumatologia 1980;18:287‐92. [PubMed] [Google Scholar]
Carrasco 1970 {published data only}
- Carrasco J. Association of a uricosuric and a uric acid inhibitor in the prophylaxis and treatment of the basis of gout. Revista Clinica Espanola 1970;117:289‐94. [PubMed] [Google Scholar]
Casademont 1968 {published data only}
- Casademont M. Results of long‐term treatment of gout using Anturane. Revista Espanola de Reumatismo y Enfermedades Osteo‐Articulares 1968;12:291‐6. [PubMed] [Google Scholar]
Cheng 2005 {published data only}
- Cheng TT, Lai HM, Chang HW, Luo SF. Elevated serum homocysteine levels for gouty patients. Clinical Rheumatology 2005;24(2):103‐6. [DOI] [PubMed] [Google Scholar]
de Gery 1974 {published data only}
- Gery A, Auscher C, Saporta L, Delbarre F. Treatment of gout and hyperuricaemia by benzbromarone, ethyl 2 (dibromo‐3,5 hydroxy‐4 benzoyl)‐3 benzofuran. Advances in Experimental Medicine & Biology 1974;41:683‐9. [DOI] [PubMed] [Google Scholar]
Delbarre 1970 {published data only}
- Delbarre F, Saporta L, Gery A, Jabre E. Treatment of gout with the combination of xanthine oxidase inhibitors and uricosuric agents. Revue du Rhumatisme et des Maladies Osteo‐Articulaires 1970;37:443‐50. [PubMed] [Google Scholar]
Dorn 1982 {published data only}
- Dorn M. The effect on uric acid and other laboratory parameters. A long‐term study. Zeitschrift fur Allgemeinmedizin 1982;58(5):296‐300. [PubMed] [Google Scholar]
Emmerson 1972 {published data only}
- Emmerson BT. The management of gout. Medical Journal of Australia 1972;1:475‐8. [DOI] [PubMed] [Google Scholar]
Erill 1975 {published data only}
- Erill S, Laporte J. Uricosuric activity of suxibuzone, a new phenylbutazone derivative. Archivos de Farmacologia y Toxicologia 1975;1(1):43‐6. [PubMed] [Google Scholar]
Famaey 1970 {published data only}
- Famaey JP, Vandenabeele G. Analysis of the hypo‐uricemic action of benzbromarone in 40 cases of gouty and non‐gouty hyperuricemia. Journal Belge de Rhumatologie et de Medecine Physique 1970;25:5‐9. [PubMed] [Google Scholar]
Fanelli 1975 {published data only}
- Fanelli GM Jr. Uricosuric agents. Arthritis & Rheumatism 1975;18:853‐8. [DOI] [PubMed] [Google Scholar]
Ferber 1980 {published data only}
- Ferber H, Bader U, Matzkies F. The action of benzbromarone in relation to age, sex and accompanying diseases. Advances in Experimental Medicine & Biology 1980;122A:287‐94. [DOI] [PubMed] [Google Scholar]
Frerick 1987 {published data only}
- Frerick H, Schaefer J, Rabinovici K, Schmidt U, Schenk N. Long‐term treatment of hyperurikemia and gout; randomised study [in German]. Therapiewoche 1987;37(36):3379‐84. [Google Scholar]
Gibson 1974 {published data only}
- Gibson T, Grahame R. Gout and hyperlipidaemia. Annals of the Rheumatic Diseases 1974;33(4):298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gresser 1994 {published data only}
- Gresser U, Gathof BS, Gross M. Benzbromarone and fenofibrate are lipid lowering and uricosuric: a possible key to metabolic syndrome?. Advances in Experimental Medicine & Biology 1994;370:87‐90. [DOI] [PubMed] [Google Scholar]
Grobner 1982 {published data only}
- Grobner W, Loffler W. Pharmacotherapy of hyperuricaemia and gout [in German]. Aktuelle Endokrinologie und Stoffwechsel 1982;3:176‐9. [Google Scholar]
Grobner 2002 {published data only}
- Grobner W, Walter‐Sack I. Hyperuricemia and gout ‐ treatment. Deutsche Medizinische Wochenschrift 2002;127:210‐3. [DOI] [PubMed] [Google Scholar]
Gunther 1968 {published data only}
- Gunther R, Herbst M, Knapp E, Siller K. On the influence of Anturan on uric acid and plasma lipids in patients with gout. Wiener Klinische Wochenschrift 1968;80:473‐8. [PubMed] [Google Scholar]
Gunther 1969 {published data only}
- Gunther R, Knapp E. Clinical findings and therapy of gout with special reference to the metabolic effects of sulfinpyrazone (Anturan) and allopurinol (Zyloric). Wiener Klinische Wochenschrift 1969;81:817‐20. [PubMed] [Google Scholar]
Gutierrez 1982 {published data only}
- Gutierrez G. Gout and crystal arthropathy [in Spanish]. Investigacion Medica Internacional 1982;9:52‐6. [Google Scholar]
Hamburger 2011 {published data only}
- Hamburger M, Baraf HSB, Adamson TC Jr, Basile J, Bass L, Cole B, et al. 2011 recommendations for the diagnosis and management of gout and hyperuricemia. Physician and Sportsmedicine 2011;39:98‐123. [DOI] [PubMed] [Google Scholar]
Hanly 2009 {published data only}
- Hanly JG, Skedgel C, Sketris I, Cooke C, Linehan T, Thompson K, et al. Gout in the elderly ‐ a population health study. Journal of Rheumatology 2009;36:822‐30. [DOI] [PubMed] [Google Scholar]
Hanvivadhanakul 2002 {published data only}
- Hanvivadhanakul P, Akkasilpa S, Deesomchok U. Efficacy of benzbromarone compared to allopurinol in lowering serum uric acid level in hyperuricemic patients. Journal of the Medical Association of Thailand 2002;85 Suppl 1:S40‐7. [PubMed] [Google Scholar]
Healey 1977 {published data only}
- Healey LA. Treatment of gout and hyperuricemia. Medical Times 1977;105:55‐60. [PubMed] [Google Scholar]
Heel 1977 {published data only}
- Heel RC, Brogden RN, Speight TM, Avery GS. Benzbromarone: a review of its pharmacological properties and therapeutic use in gout and hyperuricaemia. Drugs 1977;14:349‐66. [DOI] [PubMed] [Google Scholar]
Hertz 1972 {published data only}
- Hertz P, Yager H, Richardson JA. Probenecid‐induced nephrotic syndrome. Archives of Pathology & Laboratory Medicine 1972;94:241‐3. [PubMed] [Google Scholar]
Hollander 1968 {published data only}
- Hollander E, Schwarczmann P. Gout in advanced age. Munchener Medizinische Wochenschrift 1968;110:649‐53. [PubMed] [Google Scholar]
Houpt 1965 {published data only}
- Houpt JB. The effect of allopurinol (HPP) in the treatment of gout. Arthritis & Rheumatism 1965;8(5):899‐904. [DOI] [PubMed] [Google Scholar]
Hutchison 1971 {published data only}
- Hutchison JC, Wilkinson WH, McMahon FG. Drug‐induced hyperuricemia prevented by probenecid. Journal of Medicine 1971;2(1):45‐59. [PubMed] [Google Scholar]
Jain 1974 {published data only}
- Jain AK, Ryan JR, McMahon FG, Noveck RJ. Effect of single oral doses of benzbromarone on serum and urinary uric acid. Arthritis & Rheumatism 1974;17:149‐57. [DOI] [PubMed] [Google Scholar]
Jansen 2004 {published data only}
- Jansen TL, Reinders MK, Roon EN, Brouwers JRBJ. Benzbromarone withdrawn from the European market: another case of "absence of evidence is evidence of absence"?. Clinical & Experimental Rheumatology 2004;22:651. [PubMed] [Google Scholar]
Joseph‐Ridge 2006 {published data only}
Kawenoki‐Minc 1987 {published data only}
- Kawenoki‐Minc E. Effect of decrease in hyperuricemia levels on joint, kidney and other symptoms in patients with gout. Terapevticheskii Arkhiv 1987;59:25‐8. [PubMed] [Google Scholar]
Kersley 1966 {published data only}
- Kersley GD. Allopurinol in primary gout with and after the administration of uricosuric agents. Annals of the Rheumatic Diseases 1966;25:643‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kersley 1966a {published data only}
- Kersley GD. Treatment of gout by reduction of uric acid production. Annals of the Rheumatic Diseases 1966;25:353‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kersley 1966b {published data only}
- Kersley GD. The pharmaceutical treatment of gout. Annals of Physical Medicine 1966;8:199‐203. [DOI] [PubMed] [Google Scholar]
Kersley 1970 {published data only}
- Kersley GD. Long‐term use of allopurinol in the treatment of gout. Annals of the Rheumatic Diseases 1970;29:89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kersley 1973 {published data only}
- Kersley GD. The treatment of gout. Scottish Medical Journal 1973;18 Suppl 1:249‐50. [DOI] [PubMed] [Google Scholar]
Kim 2003 {published data only}
Klein 1968 {published data only}
- Klein G, Griendl W, Kalb R. Allopurinol in the treatment of gout. Zeitschrift fur Rheumaforschung 1968;27:455‐60. [PubMed] [Google Scholar]
Kotzaurek 1968 {published data only}
- Kotzaurek R, Hueber EF. On clinical experiences with 2‐ethyl‐3(4‐hydroxy‐3,5‐dibrombenzoyl)‐benzofuran ("Benzbromaron") in the therapy of gout and hyperuricemia. Wiener Medizinische Wochenschrift 1968;118:1014‐8. [PubMed] [Google Scholar]
Kuzell 1966 {published data only}
- Kuzell WC, Seebach LM, Glover RP, Jackman AE. Treatment of gout with allopurinol and sulphinpyrazone in combination and with allopurinol alone. Annals of the Rheumatic Diseases 1966;25:634‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee MH, Graham GG, Williams KM, Day RO. A benefit‐risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients?. Drug Safety 2008;31:643‐65. [DOI] [PubMed] [Google Scholar]
Masbernard 1976 {published data only}
- Masbernard A, Giudicelli CP. A new drug therapy for gout and hyperuricemia. Long term results. Therapeutique 1976;52:369‐74. [PubMed] [Google Scholar]
Matsumoto 2011 {published data only}
- Matsumoto K. Drug‐induced hepatic injury, the challenge for cause investigation. Rinsho Byori ‐ Japanese Journal of Clinical Pathology 2011;59:1117‐22. [PubMed] [Google Scholar]
Matzkies 1983 {published data only}
- Matzkies F. Therapy of hyperuricemia and gout. Effect of a combination therapy of allopurinol with benzbromarone. Fortschritte der Medizin 1983;101:953‐9. [PubMed] [Google Scholar]
Mertz 1969 {published data only}
- Mertz DP. Changes in the serum concentration of uric acid under the effect of benzbromaronum. Munchener Medizinische Wochenschrift 1969;111:491‐5. [PubMed] [Google Scholar]
Mertz 1976 {published data only}
- Mertz DP. Reducing the risks in the treatment of gout and hyperuricaemia (author's translation). Deutsche Medizinische Wochenschrift 1976;101(35):1288‐92. [DOI] [PubMed] [Google Scholar]
Mertz 1977a {published data only}
- Mertz DP. Therapy with allopurinol and benzbromarone, single and combined. Renal elimination of lithogenous and colloid protective substances (author's translation). Medizinische Klinik 1977;72(15):664‐8. [PubMed] [Google Scholar]
Mertz 1977b {published data only}
- Mertz DP. Isolated or combined drug therapy of hyperuricemia?. Deutsche Medizinische Wochenschrift 1977;102:1096‐7. [DOI] [PubMed] [Google Scholar]
Mertz 1983a {published data only}
- Mertz DP, Borner K. Excretion and serum concentrations of allopurinol, oxipurinol and oxipurines in combined treatment with allopurinol and benzbromarone in increasing doses. Medizinische Welt 1983;34(36):974‐9. [PubMed] [Google Scholar]
Mertz 1983b {published data only}
- Mertz DP. Current therapy: combined drug therapy of hyperuricemia, gout and alcohol induced fatty liver. Fortschritte der Medizin 1983;101:851‐3. [PubMed] [Google Scholar]
Mikkelsen 1966 {published data only}
- Mikkelsen WM, Strottman MP, Thompson GR. The effects of allopurinol on serum and urinary uric acid. Archives of Internal Medicine 1966;118:224‐8. [PubMed] [Google Scholar]
Müller 1993a {published data only}
- Müller FO, Schall R, Groenewoud G, Hundt HK, Merwe JC, Dyk M. The effect of benzbromarone on allopurinol/oxypurinol kinetics in patients with gout. European Journal of Clinical Pharmacology 1993;44(1):69‐72. [DOI] [PubMed] [Google Scholar]
Müller 1993b {published data only}
- Müller FO, Schall R, Groenewoud G, Hundt HK, Merwe JC, Dyk M. The effect of benzbromarone on allopurinol/oxypurinol kinetics in patients with gout. European Journal of Clinical Pharmacology 1993;44(1):69‐72. [DOI] [PubMed] [Google Scholar]
Nishizawa 1978 {published data only}
- Nishizawa T, Nishida Y, Masuda T, Akaoka I. Treatment of gout with alternate‐day hypo‐uricaemic drugs. Rheumatology & Rehabilitation 1978;17:143‐9. [DOI] [PubMed] [Google Scholar]
Nyfos 1967 {published data only}
- Nyfos L. Treatment of the gouty diathesis with a xanthine oxidase inhibitor. A clinical trial of allopurinol. Ugeskrift for Laeger 1967;129:253‐7. [PubMed] [Google Scholar]
Palmer 1968 {published data only}
- Palmer DG, Highton TC. A short trial assessment of uricosuric therapy in gout. Australasian Annals of Medicine 1968;17:242‐7. [DOI] [PubMed] [Google Scholar]
Peitzman 1979 {published data only}
- Peitzman SJ, Fernandez PC, Bodison W, Ellis I. Ticrynafen and probenecid in hyperuricemic, hypertensive men. Clinical Pharmacology & Therapeutics 1979;26(2):205‐8. [DOI] [PubMed] [Google Scholar]
Perez‐Ruiz 2010 {published data only}
- Perez‐Ruiz Fernando, Hernandez‐Baldizon Samuel, Herrero‐Beites Ana M, Gonzalez‐Gay Miguel A. Risk factors associated with renal lithiasis during uricosuric treatment of hyperuricemia in patients with gout. Arthritis care & research 2010;62(9):1299‐305. [DOI] [PubMed] [Google Scholar]
Podevin 1967 {published data only}
- Podevin R, Paillard F, Amiel C, Richet G. Effect of benziodarone on the renal excretion of uric acid. Revue Francaise d Etudes Cliniques et Biologiques 1967;12(4):361‐7. [PubMed] [Google Scholar]
Rausch‐Stroomann 1965 {published data only}
- Rausch‐Stroomann JG. Benemid in the treatment of primary and secondary gout. Medizinische Welt 1965;45:2555‐61. [PubMed] [Google Scholar]
Reinders 2007 {published data only}
- Reinders MK, Roon EN, Houtman PM, Brouwers JRBJ, Jansen TL. Biochemical effectiveness of allopurinol and allopurinol‐probenecid in previously benzbromarone‐treated gout patients. Clinical Rheumatology 2007;26(9):1459‐65. [DOI] [PubMed] [Google Scholar]
Rice 1959 {published data only}
- Rice FA. Probenecid (B enemid) in the treatment of gout and gouty arthritis. Minnesota Medicine 1959;42:209‐14. [PubMed] [Google Scholar]
Scott 1966b {published data only}
- Scott JT, Hall AP, Grahame R. Allopurinol in treatment of gout. British Medical Journal 1966;2(5509):321‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1968 {published data only}
- Scott JT, O'Brien PK. Probenecid, nephrotic syndrome, and renal failure. Annals of the Rheumatic Diseases 1968;27:249‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seebach 1967 {published data only}
- Seebach LM. Allopurinol in the treatment of gout. Lancet 1967;87:411‐4. [PubMed] [Google Scholar]
Serre 1970 {published data only}
- Serre H, Simon L, Claustre J. Uric acid inhibitors in the treatment of gout. Apropos of 126 cases [in French]. La Semaine des Hopitaux 1970;46:3295‐301. [PubMed] [Google Scholar]
Sjoberg 1966 {published data only}
- Sjoberg KH. Allopurinol therapy of gout with renal complications. Annals of the Rheumatic Diseases 1966;25:688‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sternon 1967 {published data only}
- Sternon J, Kocheleff P, Couturier E, Balasse E, Vanden Abeele P. Hypouricemizing effect of benzbromarone. Study of 24 cases (preliminary results). Acta Clinica Belgica 1967;22:285‐93. [DOI] [PubMed] [Google Scholar]
Sutaria 2006 {published data only}
- Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout ‐ a systematic review. Rheumatology 2006;45:1422‐31. [DOI] [PubMed] [Google Scholar]
Weinberger 1978 {published data only}
- Weinberger A, Pinkhas J. The efficacy of treatment with allopurinol versus uricosuric drugs in gout. Panminerva Medica 1978;20:155‐6. [PubMed] [Google Scholar]
Wortmann 2005 {published data only}
- Wortmann RL, Schumacher HR Jr, Lewis CS Jr. Monosodium urate deposition arthropathy part II: treatment and long‐term management of patients with gout. Advanced Studies in Medicine 2005;5(4):183‐94. [Google Scholar]
Xie 2007 {published data only}
- Xie J‐Y, Wang L, Li Q‐X, Li X‐M. Study on mechanisms of electroacupuncture treatment of acute gouty arthritis. Zhongguo Zhenjiu 2007;27(12):898‐900. [PubMed] [Google Scholar]
Yamamoto 1997 {published data only}
- Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Yamakita J, Higashino K. Effect of allopurinol and benzbromarone on the concentration of uridine in plasma. Metabolism: Clinical & Experimental 1997;46:1473‐6. [DOI] [PubMed] [Google Scholar]
Yonetsugu 1971 {published data only}
- Yonetsugu Fujita. Uricosuric Effect of Yagifraxin Tablet in Gout: Comparison with Sulfinpyrazone. Clinical Report: Kiso to Rinsho 1971;5(11):1731‐7. [Google Scholar]
Zhang 2006 {published data only}
- Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases 2006;65:1312‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zollner 1970 {published data only}
- Zollner N, Griebsch A, Fink JK. Effect of benzbromarone on serum uric acid level and uric acid excretion of patients with gout. Deutsche Medizinische Wochenschrift 1970;95:2405‐12. [DOI] [PubMed] [Google Scholar]
Additional references
Campion 1987
- Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. American Journal of Medicine 1987;82(3):421‐6. [DOI] [PubMed] [Google Scholar]
Cates 2008
- Cates C. Visual Rx Version 3. www.nntonline.net/visualrx/ (accessed 28 September 2014).
Dalbeth 2011
- Dalbeth N, Schauer C, MacDonald P, Perez‐Ruiz F, Schumacher HR, Hamburger S. Methods of tophus assessment in clinical trials of chronic gout: a systematic literature review and pictorial reference guide. Annals of the Rheumatic Diseases 2011;70(4):597‐604. [DOI] [PubMed] [Google Scholar]
DeAngelis 2004
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292(11):1363‐4. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hagos 2007
- Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. Journal of the American Society of Nephrology 2007;18(2):430‐9. [DOI] [PubMed] [Google Scholar]
Hautekeete 1995
- Hautekeete ML, Henrion J, Naegels S. Severe hepatotoxicity related to benzarone: a report of three cases with two fatalities. Liver (Copenhagen) 1995;15(1):25‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmes 1985
- Holmes EW. Clinical gout and the pathogenesis of hyperuricemia. Arthritis and Allied Conditions. 10th Edition. Philadelphia: Lea & Febiger, 1985:1445. [Google Scholar]
Khanna 2011
- Khanna D, Sarkin AJ, Khanna PP, Shieh MM, Kavanaugh A, Terkeltaub R, et al. Minimally important differences of the gout impact scale in a randomized controlled trial. Rheumatology 2011;50(7):1331‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khanna 2012
- Khanna D, FitzGerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care & Research 2012;64(10):1431‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Ruiz 2002
- Perez‐Ruiz F, Calabozo M, Pijoan JI, Herrero‐Beites AM, Ruibal A. Effect of urate‐lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis & Rheumatism 2002;47(4):356‐60. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schumacher 2009
- Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. Journal of Rheumatology 2009;36(10):2342‐5. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seth 2013
- Seth RS, Kydd AS, Buchbinder R, Bombardier C, Edwards CJ. Allopurinol for chronic gout. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD006077.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2011
- Shin HJ, Takeda M, Enomoto A, Fujimura M, Miyazaki H, Anzai N, et al. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology 2011;16(2):156‐62. [DOI] [PubMed] [Google Scholar]
Shoji 2004
- Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis & Rheumatism 2004;51(3):321‐5. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Taylor WJ, Simon LS, Khanna PP, Stamp LK, McQueen FM, et al. Patient‐reported outcomes in chronic gout: a report from OMERACT 10. Journal of Rheumatology 2011;38(7):1452‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2010
- Smith EUR, Diaz‐Torne C, Perez‐Ruiz F, March LM. Epidemiology of gout: an update. Best Practice Research in Clinical Rheumatology 2010;24(6):811‐27. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stocker 2008
- Stocker SL, Williams KM, McLachlan AJ, Graham GG, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in healthy subjects. Clinical Pharmacokinetics 2008;47(2):111‐8. [DOI] [PubMed] [Google Scholar]
Stocker 2011
- Stocker SL, Graham GG, McLachlan AJ, Williams KM, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in patients with gout. Journal of Rheumatology 2011;38(5):904‐10. [DOI] [PubMed] [Google Scholar]
Takahashi 2003
- Takahashi S, Moriwaki Y, Yamamoto T, Tsutsumi Z, Ka T, Fukuchi M. Effects of combination treatment using anti‐hyperuricaemic agents with fenofibrate and/or losartan on uric acid metabolism. Annals of the Rheumatic Diseases 2003;62(6):572‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tayar 2012
- Tayar JH, Lopez‐Olivo MA, Suarez‐Almazor ME. Febuxostat for treating chronic gout. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008653.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 1977
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü T‐F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis & Rheumatism 1977;20:895‐900. [DOI] [PubMed] [Google Scholar]
Zhu 2011
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007‐2008. Arthritis & Rheumatism 2011;63(10):3136‐41. [DOI] [PubMed] [Google Scholar]


