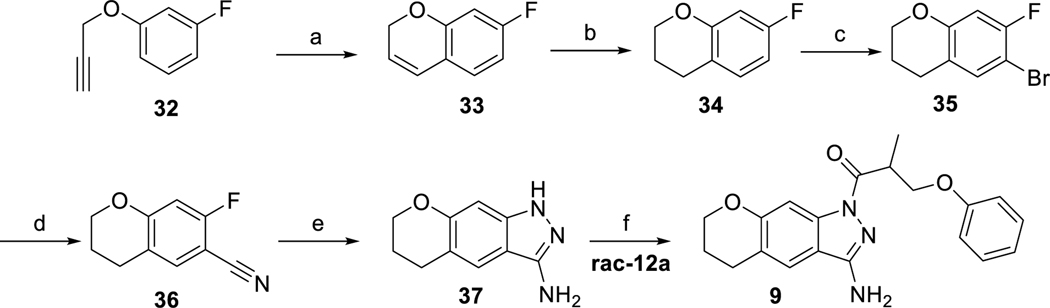
Scheme 8.
Synthesis of the fused acylindazole derivative (a) (acetonitrile)[2-biphenyl)di-tert-butylphosphine]gold(I) hexafluoroantimonate, toluene, 0 °C (crude); (b) H2/Pd(OH)2/C, MeOH, rt, 2 h (crude); (c) N-bromosuccinimide, CH3CN, 0 °C (23% over three steps); (d) 1. BuLi, THF, −79 °C. 2. dimethylmalononitrile, THF, −79 °C (54%); (e) N2H4, BuOH, 120 °C, 22 h (76%); (f) EDC × HCl, HOAt, DMF, microwave irradiation (61%).