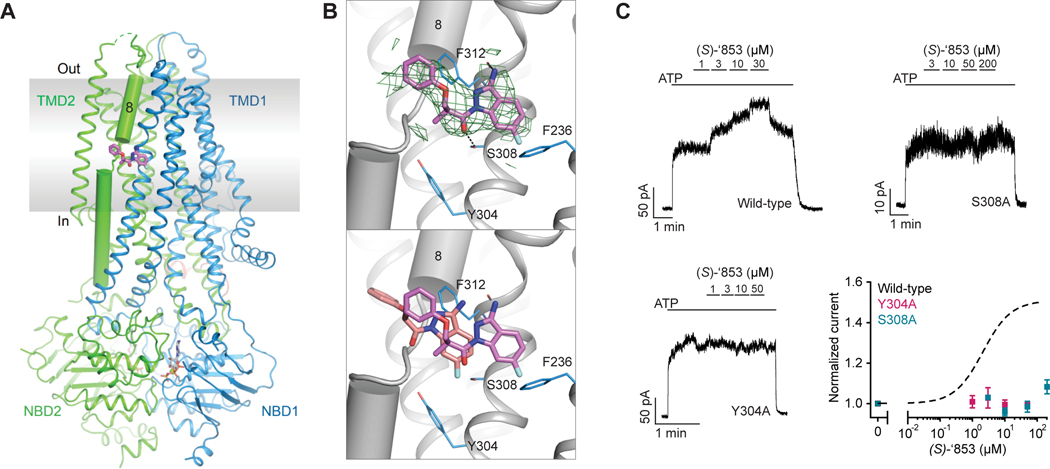
Figure 3: Z1834339853 binds to the same site as ivacaftor and GLPG1837.
(A) Cryo-EM structure of phosphorylated and ATP-bound CFTR (E1371Q) in complex with ‘853. (B) Zoomed-in views of the density of ‘853 (top) and a comparison between the docked pose (salmon) and the cryo-EM pose (magenta). Compared to the experimentally determined pose, the docked ‘853 shifted towards R933. This difference is likely due to the presence of an unknown density between R933 and the potentiator (Liu et al., 2019), which was not modeled for docking. (C) Representative macroscopic current traces and dose-response curves of fully phosphorylated WT, S308A, and Y304A CFTR in response to perfusion of (S)-’853 onto inside-out excised membrane patches. 3 mM ATP was used. Each data point represents the mean and SEs determined from 3 to 12 patches.