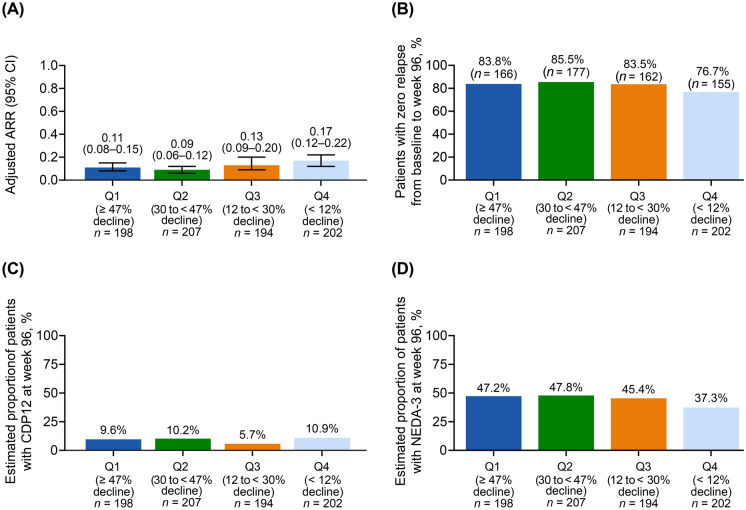
Fig. 3.
a Adjusted ARRa on treatment, b patients with no relapses from baseline to week 96, c estimated proportion of patients with CDP12 at week 96, and d estimated proportion of patients with NEDA-3 at week 96, stratified by % ALC decline from baseline to week 96b. aCalculation of ARR was based on patient-reported relapses in the 12 months before study entry compared with protocol-defined relapses during the study period. Adjusted ARR was based on a Poisson regression model adjusted for treatment duration. bPatients were stratified into quartiles based on the decline in ALC from baseline to week 96. The four quartiles used were Q1 (≥ 47% ALC decline from baseline); Q2 (30% to < 47% ALC decline from baseline); Q3 (12% to < 30% ALC decline from baseline); Q4 (< 12% ALC decline from baseline). ALC absolute lymphocyte count, ARR annualized relapse rate, CDP12 confirmed disability progression sustained for 12 weeks per EDSS, CI confidence interval, EDSS expanded disability status score, NEDA-3 no evidence of disease activity-3, Q quartile