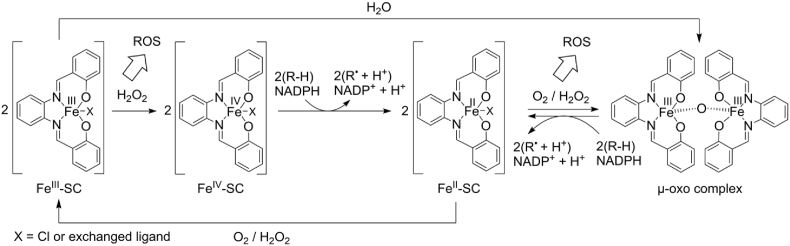
Fig. 6.
Proposed catalytic mechanism of SCs for the (per)oxidation of phospholipid-bound PUFAs and redox cofactors. Initiation: H2O2 oxidizes FeIII in SCs to a hyperoxidative FeIV state, allowing one- or two-electron oxidations of redox substrates, such as TMB or endogenous factors, including the allylic positions of PUFAs in membrane phospholipids (R–H) or NADPH, thereby yielding FeII-SC. NADPH is a central cofactor in the control of redox homeostasis that keeps ferroptosis at bay, but also has pleiotropic other functions, some of which are related to cell death programs other than ferroptosis. Alternatively, FeIII–SCs may spontaneously dimerize to a minor extent in aqueous solutions to form μ-oxo complexes [29]. Propagation: FeII-SC then undergoes a redox cycle initiated by the oxidation to μ-oxo complexes with a binuclear FeIII center, which in turn allows one- or two-electron oxidation of redox substrates in the course of regenerating monomeric FeII-SC. Reactive oxygen species (not necessarily OH-radicals) are formed from O2 or H2O2 during these multiple oxidation steps, which also involve the formation of secondary radicals.