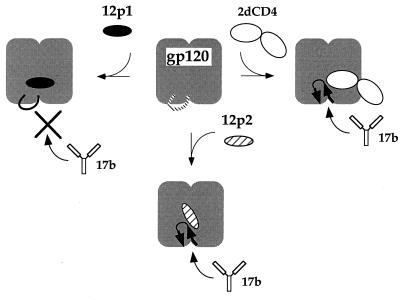
FIG. 7.
Model for the relationship of peptide, CD4, and MAb 17b binding sites. The diagram is based on the recent crystal structure of the ternary complex of gp120-2dCD4-Fab 17b (13). The two domains of gp120 are represented by the bilobal icon. These domains are linked by the bridging sheet (loop with arrows) in the CD4-bound state, which is probably not a stable structure in the absence of CD4 (dashed line). CD4 binding to gp120 stabilizes the sheet and induces the formation of a site for MAb 17b (top right). Thus, CD4 binding enhances the affinity for this MAb. We propose that peptide 12p1 binds near the CD4 site and inhibits MAb 17b binding by preventing the formation of the bridging-sheet structure (top left) whereas peptide 12p2 binds at a site overlapping that of 12p1 but stabilizes the bridging sheet (bottom). Thus, like CD4, 12p2 enhances the affinity of MAb 17b.