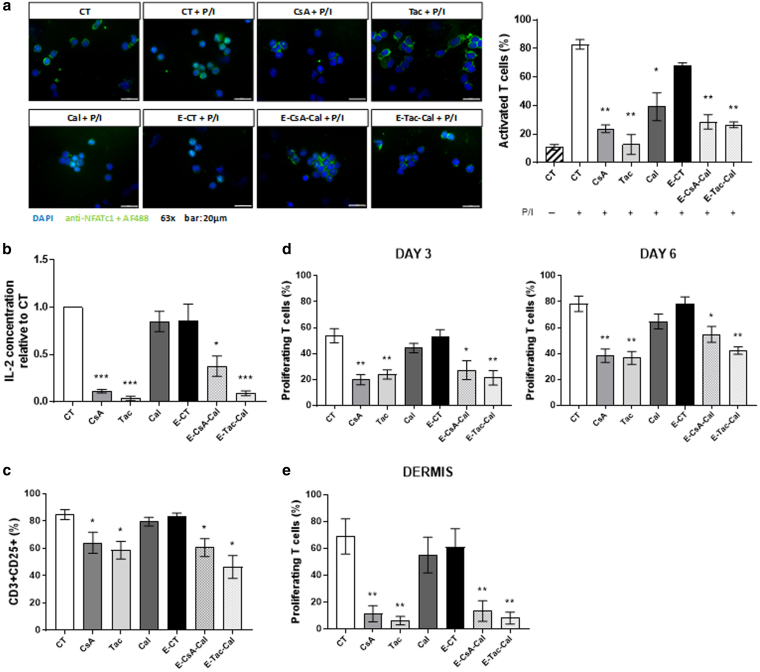
Figure 1.
Effects of Em on healthy T cells.(a) Quantification of NFAT translocation with representative immunofluorescent images of each condition in cytospined human PBMCs activated for 30 minutes with P/I. At least 500 cells were counted for each donor (n = 3 independent human individuals). Bar = 20 μm. Pretreated PBMCs were tested for their ability to (b) produce IL-2, (c) express CD25 at their surface, and (d) proliferate after 3 and 6 days of activation with CD3xCD28 microbeads. (e) Quantification of human dermal T-cell proliferation by flow cytometry analysis of CFSE staining after 7 days of activation with OKT3 mAb directed against CD3. (f) Comparison of control emulsion without active substances (denoted as E-CT), emulsions associating CsA- or Tac-loaded NPs with Cal (E-CsA-Cal or E-Tac-Cal, respectively), or emulsions including only 1 AS (E-CsA, E-Tac, and E-Cal), besides the AS solution described as CsA, Tac, or Cal and control culture medium (denoted as CT) on proliferation of human PBMCs expressed as mean percentage of each successive generation of live cells, from M1 (nonproliferating cells) to M7 (after 6 cell divisions) after 6 days of activation with CD3xCD28 microbeads. Results represent the mean ± SD of 3 independent experiments in a and the mean ± SEM of 6 independent experiments in b–e. Statistical comparison was assessed using the Wilcoxon rank-sum test. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. Cal, calcitriol; CFSE, carboxyfluorescein succinimidyl ester; CsA, cyclosporin A; Em, pickering emulsion; NFAT, nuclear factor of activated T cell; NP, nanoparticle; P/I, phorbol myristate acetate/ionomycin; PMA, phorbol myristate acetate; Tac, tacrolimus.