FIGURE 3.
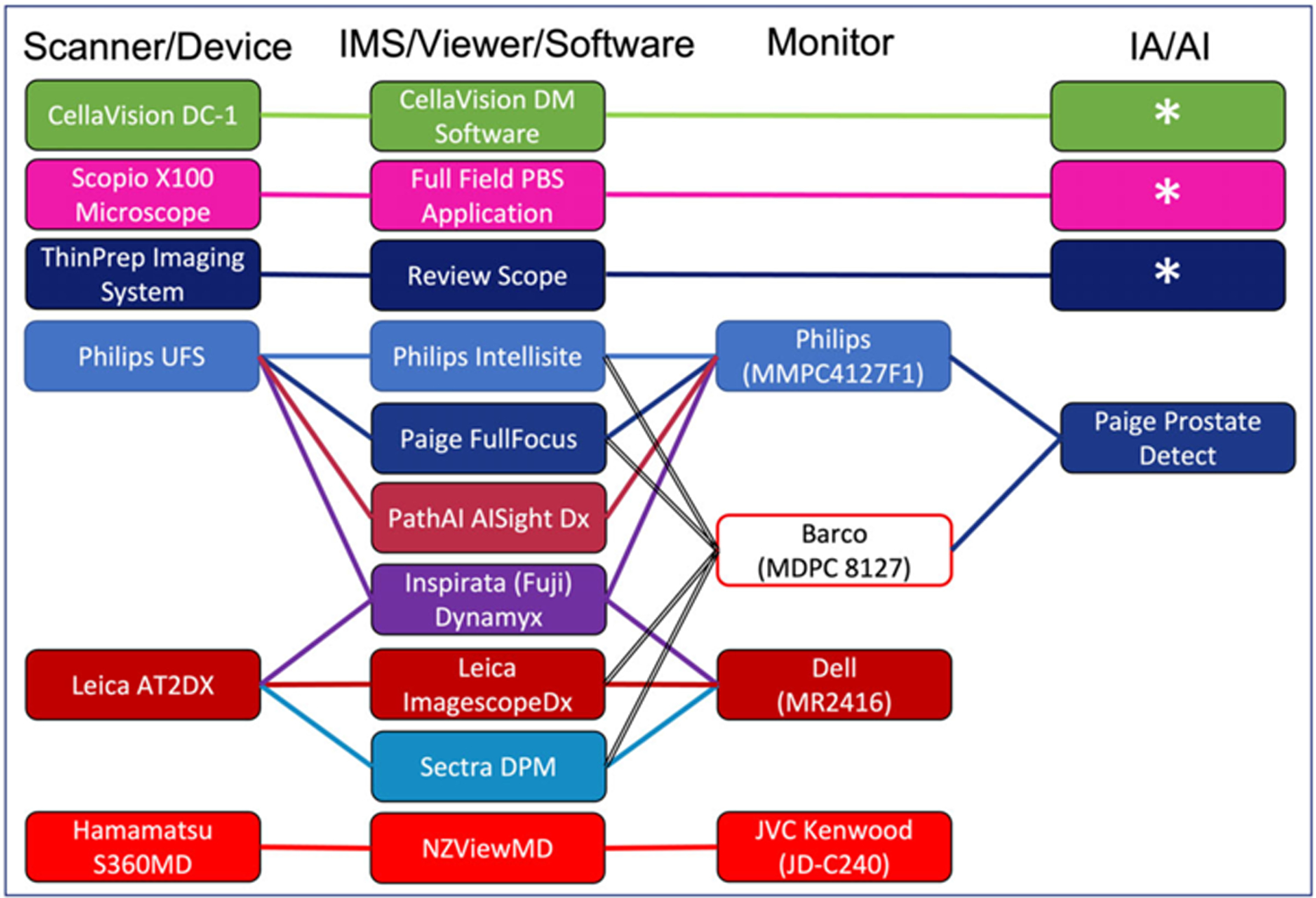
Regulatory authorized digital pathology systems. Represents the current Food and Drug Administration (FDA) authorized digital pathology systems as of April 12, 2023. Asterisks (*) represent FDA authorized devices that incorporate image analysis/machine learning as part of their integrated solution. AI, artificial intelligence; DPM, digital pathology module; IA, image analysis; IMS, image management system; PBS, peripheral blood smear
