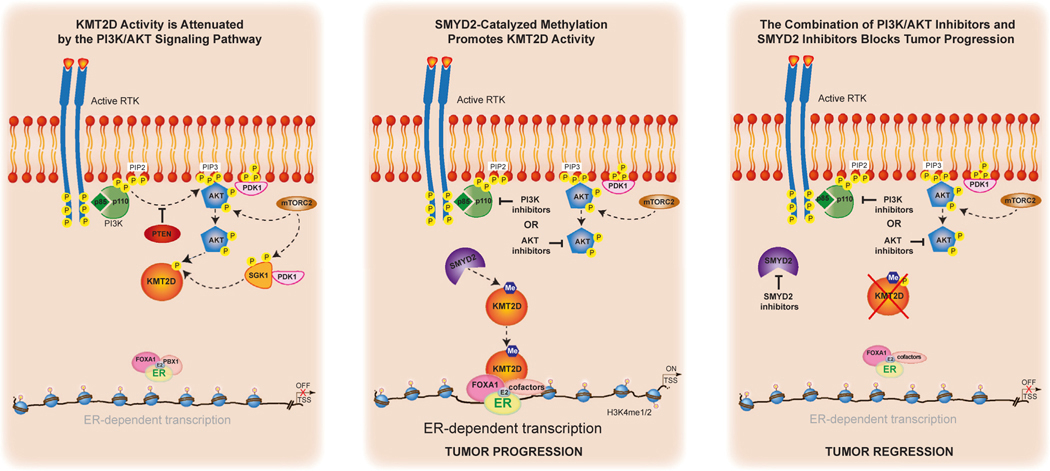
Figure 6. Proposed model.
We had previously uncovered a critical role for the lysine methyltransferase KMT2D in regulating the interplay between ER and the PI3K/AKT pathway in breast cancer through the direct phosphorylation of KMT2D by the PI3K effectors AKT/SGK4,5 (first panel). AKT/SGK negatively regulate KMT2D through direct S1331 phosphorylation. When PI3K is inhibited, this downstream regulatory event is lost, leading to increased chromatin recruitment of KMT2D and an upregulation of ER-dependent transcription.4,5 Here we discovered a previously unknown regulatory mechanism for KMT2D function mediated by the direct methylation of KMT2D by the lysine methyltransferase SMYD2 (middle panel). Ablation of SMYD2 abrogates alpelisib-induced chromatin binding of KMT2D genome-wide and at ER cis-regulatory elements. Accordingly, loss of SMYD2 attenuates alpelisib-induced gene expression changes, including ER transcriptional output. Knockdown or pharmacological inhibition of SMYD2 sensitizes breast cancer cells, patient-derived organoids, and tumors to PI3K/AKT inhibition and endocrine therapy through KMT2D K1330 methylation (last panel).