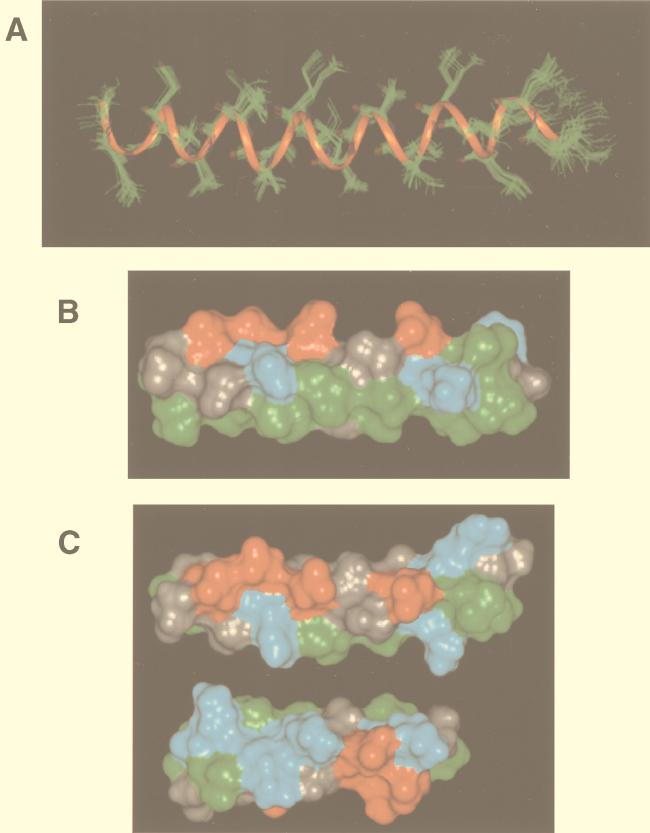
FIG. 8.
Representations of the structure of NDVFhr24 determined by NMR. (A) Twenty randomly selected structures of NDVFhr24 from simulated-annealing calculations using interproton distance and intramolecular hydrogen bond data as described in Materials and Methods superimposed showing backbone residues only. A ribbon is shown through the backbone atoms to highlight the helical structure. (B) Representative structure of NDVFhr24 showing solid surfaces. The hydrophobic face of the peptide is shown along the bottom of the structure. Green indicates hydrophobic residues, red indicates acidic residues, and blue indicates basic residues. (C) The top structure is a representative structure of NDVFhr24 (HR1) showing solid surfaces. The charged face of the helix is shown. The bottom structure shows the charged face of the NDVFz20 (HR2) peptide. Rotation of both peptides along the long axis and toward the center would result in alignment of the positive charges with the negative charges.