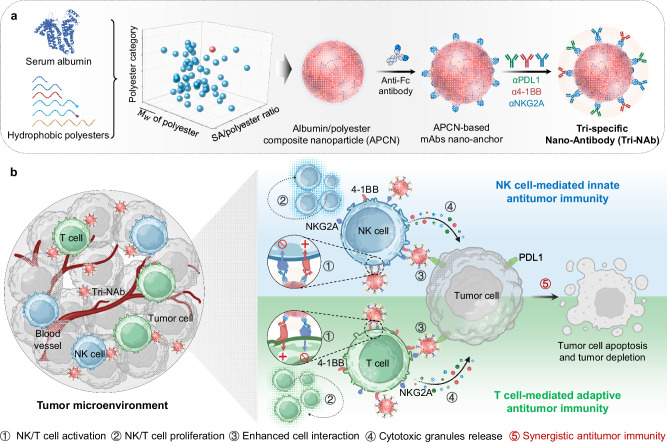
Fig. 1. Schematic diagram illustrating the construction of the Tri-NAb and its proposed mechanism of action to potentiate both innate and adaptive antitumor immune responses.
a Initially, a biocompatible albumin/polyester composite nanoparticle (APCN) with the optimal formulation was meticulously engineered through multivariate screening. Subsequently, the tri-specific Nano-Antibody (Tri-NAb) was acquired by immobilizing αPDL1/α4-1BB/αNKG2A onto APCN preassembled with αFc (APCN@NA). b Following administration, the Tri-NAb accumulated at the tumor site, effectively triggering the activation (①) and proliferation (②) of natural killer (NK) and CD8+ T cells while augmenting their interactions with tumor cells (③), thereby stimulating the release of cytotoxic granules (④). This orchestrated interplay culminated in the induction of efficient tumor cell apoptosis (⑤), harnessing synergistic effects of innate and adaptive immunity mediated by NK and CD8+ T cells. Figure 1b was created with BioRender.com under a CC-BY-NC-ND license.