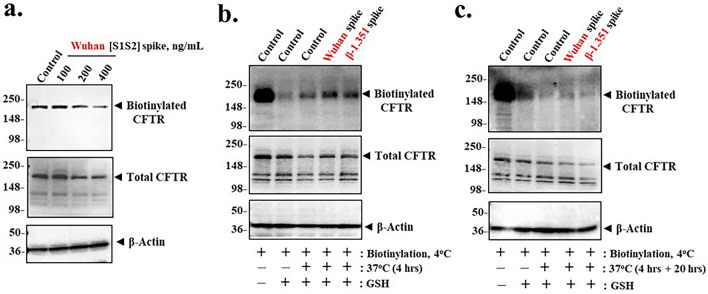
Figure 6.
Spike proteins induce loss of cell surface CFTR expression by inhibiting endosomal recycling in differentiated BCi.NS1.1 (d-BCi) epithelia. (a) ALI differentiated dBCi cells were incubated apically with different concentrations of Wuhan-Hu-1 [S1S2] spike protein for 4 h, washed and then incubated for additional 20 h under ALI conditions. After completion of incubation, apical membrane expression of CFTR was determined by cell surface biotinylation using cell impermeable Sulfo-NHS-SS-biotin. Representative Western blot images of cell surface and total CFTR expression from three independent experiments are shown. β-actin was used for equal loading of protein (b) Recycling assay of endogenous CFTR after 4-h incubation with spike protein. ALI differentiated dBCi cells were first biotinylated at 4 °C with Sulfo-NHS-SS-biotin for 1 h, followed by incubation at 37 °C for 4 h apically with media control or either 400 ng/mL Wuhan-Hu-1 [S1S2] or β-1.351 [S1S2] spike proteins. After incubation, biotin molecules remaining at the cell surface were stripped with glutathione (GSH). (c) Recycling assay of endogengous CFTR after a total 24-h incubation. Cell surface biotinylation and treatments were carried out as described in part (b), except that after, 4-h incubation with media or spike protein apically, dBCi cells were further incubated at 37 °C for additional 20 h, followed by GSH stripping. Biotinylated and total CFTR pools in parts (b) and (c) were analyzed by Western blotting. Cell lysate samples (lane 1) and samples treated with GSH (lane 2) were used as positive controls for biotinylation and GSH stripping processes, respectively. β-actin was used for equal loading of protein. Western analyses represent the results of three independent experiments.