FIGURE 3.
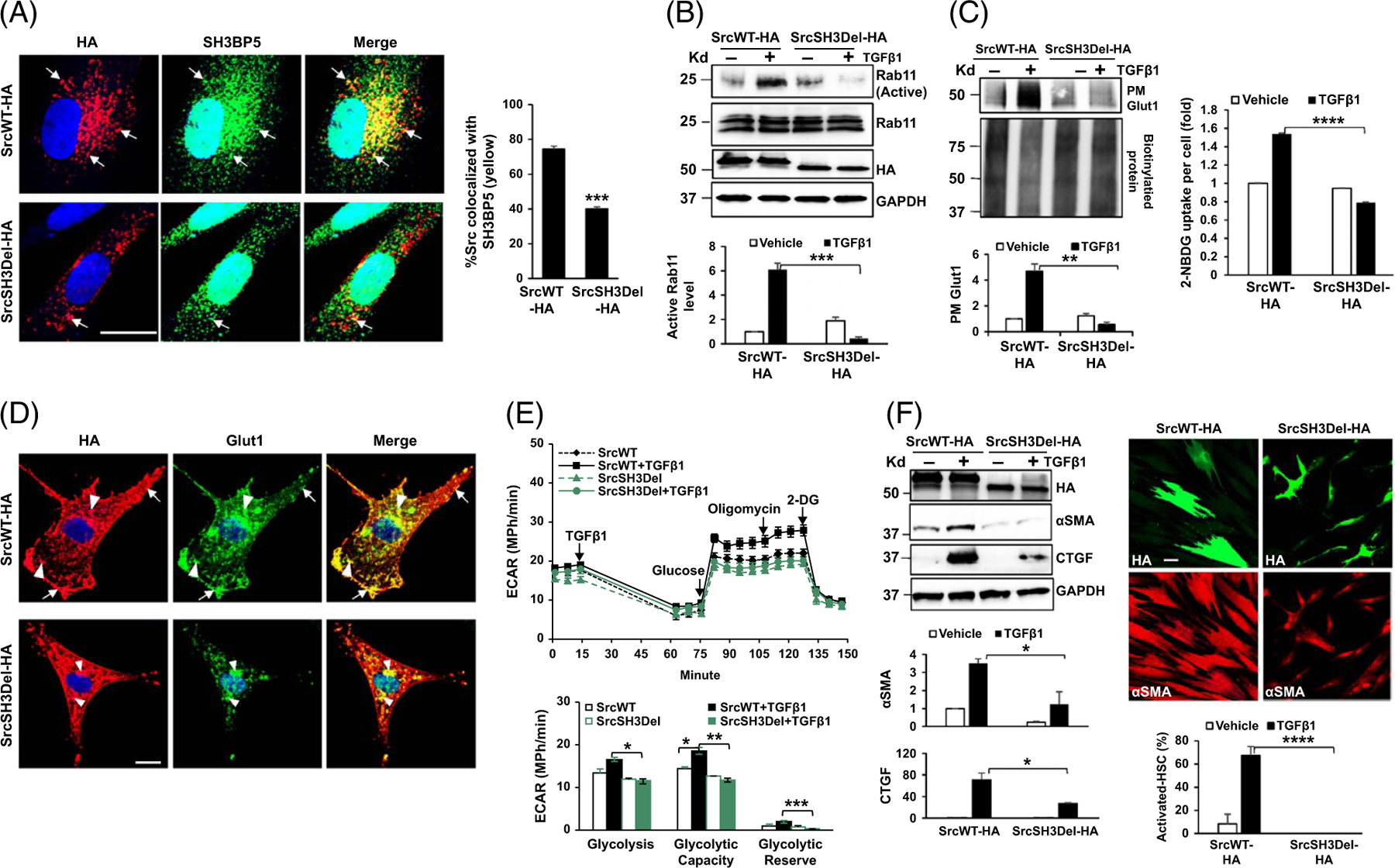
The SH3 domain of Src is required for Rab11 activation, PM Glut1, glycolysis, and HSC activation induced by TGFβ1. (A) HSCs expressing SrcWT-HA or SrcSH3Del-HA were collected for double IF for HA (red) and SH3BP5 (green). Deleting the Src SH3 domain reduced Src/SH3BP5 colocalization in HSCs. ***p < 0.001 by t-test, n = 15 cells. Bar, 20 μm. (B) Rab11 activity assay revealed that TGFβ1 promoted Rab11 activation in SrcWT-HA–expressing cells, but not in SrcSH3Del-HA–expressing cells. ***p < 0.001 by ANOVA, n = 3 repeats. (C) Left, biotinylation assay showed that PM Glut1 was increased by TGFβ1 in SrcWT-HA–expressing cells, but not in SrcSH3del-HA–expressing cells. **p < 0.01 by ANOVA, n = 3 repeats. Right, the Glucose uptake assay revealed that glucose uptake induced by TGFβ1 was abolished by the SrcSH3del-HA mutant. ****p < 0.0001 by ANOVA, n = 20,000. (D) Double IF and confocal microscopy demonstrated evident colocalization of Glut1 (green) and SrcWT-HA (red) at the endosomes (arrowheads) and PM of HSCs (arrows), and diminished colocalization of Glut1 (green) and SrcSH3Del-HA (red) at the PM. Bar, 20 μm. (E) Real-time ECAR data revealed that TGFβ1-promoted glycolysis was suppressed by the SrcSH3del-HA mutant. *p < 0.05; **p < 0.01 by ANOVA, n = 5. F. WB (left) and αSMA IF (red, right) for HSC activation markers showed that TGFβ1-induced HSC activation was suppressed by the SrcSH3del-HA mutant. *p < 0.05; ****p < 0.0001 by ANOVA, n = 3 for WB and n = 10 microscopic fields per group for IF. Bar, 50 μm. Abbreviations: αSMA, alpha-smooth muscle actin; ECAR, extracellular acidification rate; Glut1, glucose transporter 1; HA, human influenza hemagglutinin; IF, immunofluorescence; PM, plasma membrane; SH3BP5, SH3 domain–binding protein 5; SrcSH3Del-HA, HA-tagged Src SH3 domain deletion mutant; SrcWT-HA, HA-tagged wild-type Src; TGFβ1, transforming growth factor-beta 1; WB, western blot.
