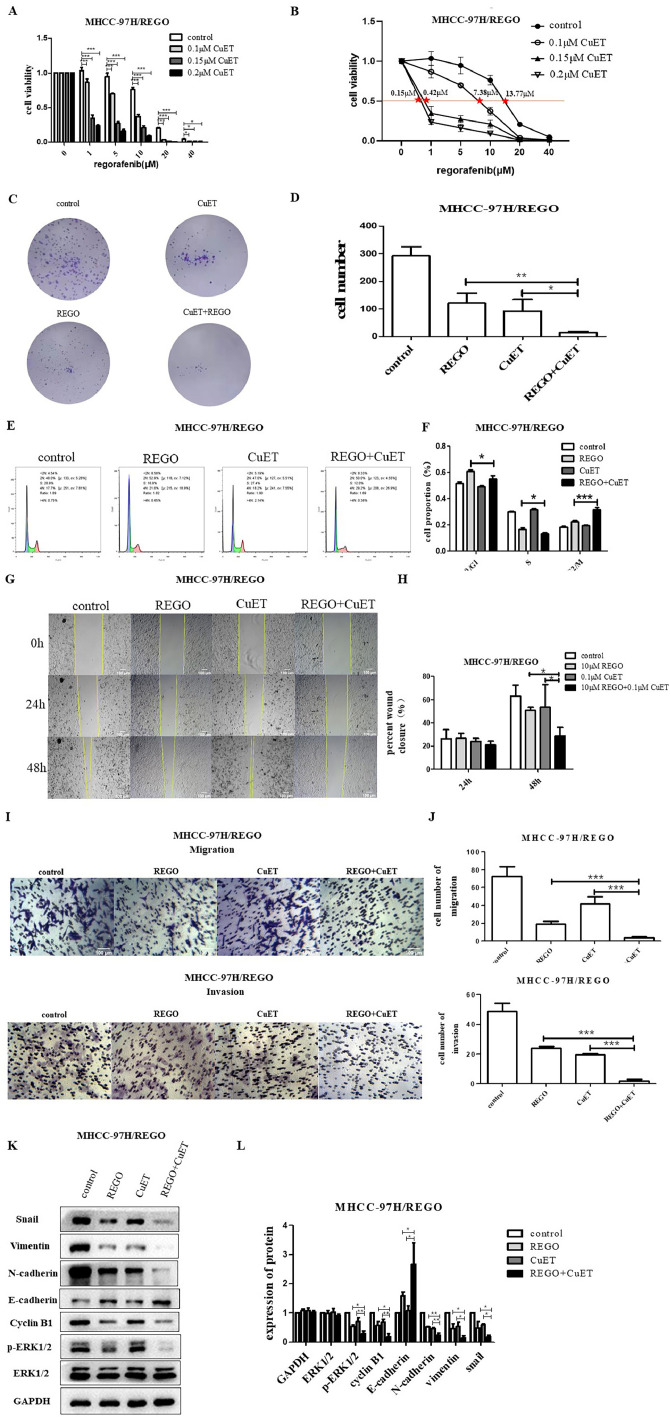
Fig. 5.
Effect of CuET on the regorafenib sensitivity of regorafenib-resistant HCC cells in vitro.
A. Viability of MHCC-97H/REGO cells cocultured with the indicated concentrations of CuET (0 μM, 0.1 μM, 0.15 μM, 0.2 μM), the indicated concentrations of regorafenib (0 μM, 1 μM, 5 μM, 10 μM, 20 μM, 40 μM) or a combination of the two for 24 h, as determined by the CCK-8 assay. B. The IC50 values of MHCC-97H/REGO cells presented in A are marked (control-13.77 μM, 0.1 μM CuET-7.38 μM, 0.15 μM CuET-0.42 μM, 0.2 μM CuET-0.15 μM). C-D. Colony formation assay on MHCC-97H/REGO cells. Cells were plated at a density of 500 cells per well and then treated with 0.1 μM CuET, 10 μM regorafenib, or a combination of 0.1 μM CuET and 10 μM regorafenib for 24 h. Quantitative analysis was performed. E-F. Cell cycle distributions of control, regorafenib, CuET, or the combination (same drug concentrations as in Figure C) -treated MHCC-97H/REGO cells. Quantitative analysis was subsequently performed. G-H. Wound healing assay of MHCC-97H/REGO cells after treatment with the control, regorafenib, CuET, or the combination (same drug concentrations as in Figure C) for 0 h, 24 h and 48 h. Quantitative analysis of the cell healing rate was performed. I-J. Transwell assay of MHCC-97H/REGO cells at 8 × 104 cells per well. Cells were treated as presented in Figure C before being plated in the Transwell chamber. Quantitative analysis was subsequently performed. K-L. Qualitative and quantitative analyses of the expression levels of proliferation markers and the EMT markers ERK and p-ERK by western blotting. *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA).