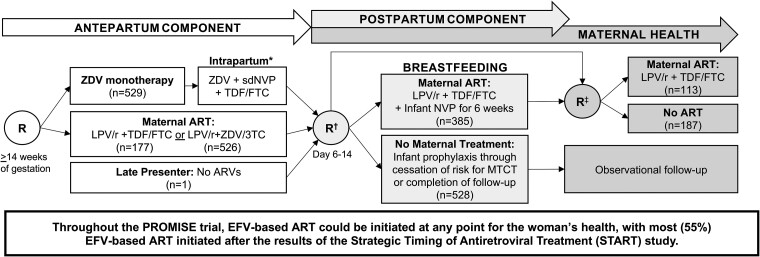
Figure 1.
Promoting Maternal and Infant Survival Everywhere (PROMISE) trial randomization schema for efavirenz-based antiretroviral therapy (ART) substudy participants. The timing of all 3 antiretroviral drug randomizations are shown for each “component” of the PROMISE trial. The number of women in each PROMISE randomization group is shown in parentheses. Participants in this substudy cohort (N = 1233) had efavirenz-based ART prescribed (usually as tenofovir + lamivudine [or emtricitabine] + efavirenz) as part of local standard of care at any phase of the PROMISE trial. The impact of pre-efavirenz-based ART HIV drug resistance on ART suppression was assessed in women prescribed efavirenz-based ART at any phase of the PROMISE study. *Single-dose nevirapine was given at labor/delivery with a “tail” of tenofovir disoproxil fumarate /emtricitabine for 6–14 days to reduce the risk of resistance. †Eligible and willing antepartum and late-presenting mothers and their infants were randomized for the duration of breastfeeding; infants were to be followed to 104 weeks of age. Some mothers who were ineligible for the postpartum component were directly randomized to the maternal health component after delivery. ‡Randomization to the maternal health component occurred at breastfeeding cessation or at or after 74 weeks of breastfeeding for women randomized in the postpartum component. Those randomized directly to the maternal health component after delivery were randomized between 6 and 28 days postpartum. Participants randomized in the antepartum component and not randomized in the postpartum component remained in observational follow-up. Abbreviations: 3TC, lamivudine; ART, antiretroviral treatment; ARVs, antiretroviral drugs; EFV, efavirenz; FTC, emtricitabine; LPV/r, ritonavir-boosted lopinavir; MTCT, mother-to-child transmission; NVP, nevirapine; PROMISE, Promoting Maternal and Infant Survival Everywhere; R, randomization; sdNVP, single-dose nevirapine; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.