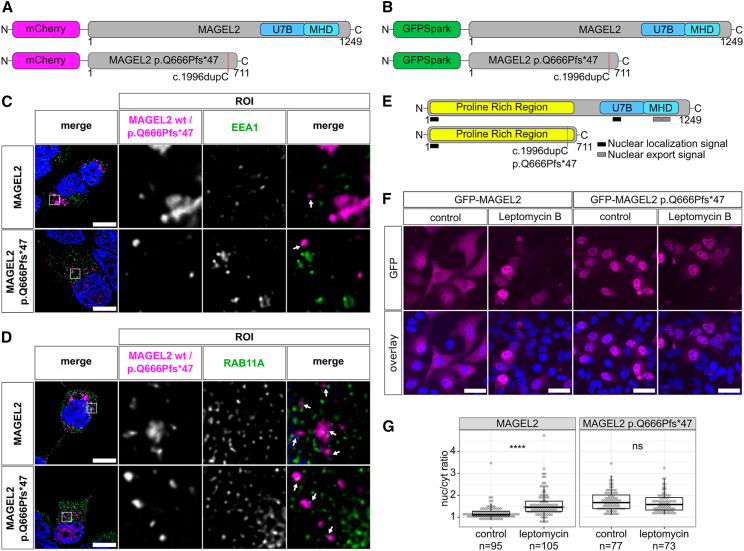
Figure 1.
Subcellular localization of MAGEL2 WT and p.Gln666Profs∗47
(A and B) MAGEL2/p.Gln666Profs∗47 fusion proteins employed for investigation of their subcellular localization, tagged with either an N-terminal mCherry (A) or GFPspark (B).
(C and D) Confocal immunofluorescence microscopy of recombinant fusion proteins WT mCherry-MAGEL2 or mCherry-p.Gln666Profs∗47 (magenta) in transiently transfected HEK293T cells, 24 h after transfection. Nuclei were counterstained with DAPI (blue). The scale bar is 10 μm. White arrows indicate sites of spatial proximity between WT MAGEL2 and p.Gln666Profs∗47 with endogenous early endosomes (C) and recycling endosomes (D), which were visualized with anti-EEA1 and anti-RAB11A antibodies (green), respectively. At least 5 cells were imaged in three independent replicates and representative cells are depicted.
(E) MAGEL2 WT and truncated p.Gln666Profs∗47 with computationally predicted putative NLS (black boxes) and NES (gray boxes) motifs.
(F and G) HeLa cells were transfected with the GFP-tagged wild-type MAGEL2 or MAGEL2 c.1996dupC mutant. 48 h after transfection cells were treated with leptomycin B (20 nM) or left untreated for 20 h. Nuclei were counterstained with DAPI (blue).
(F) HeLa cells with and without leptomycin B treatment. Cells from three independent transfection experiments were used for quantification. The scale bar is 40 μm.
(G) Quantification of the nuclear/cytosolic ratio of individual cells from three independent experiments. Wilcoxon tests were calculated employing rstatix.