Figure 1.
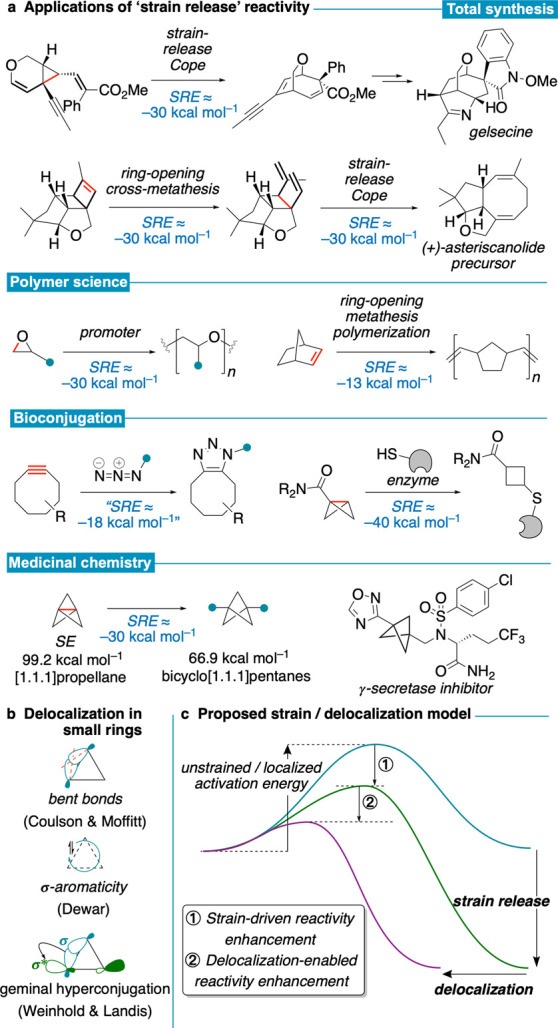
Ring strain in organic chemistry. (a) Examples of strain release-driven reactivity, including total synthesis,16,17 bioconjugation reactions,7,8 ring-opening polymerization,18 and bioisostere synthesis.19 (b) Ground state models for electron delocalization in three-membered rings. (c) This work: strain release and delocalization combine to enhance reactivity through lower activation barriers and earlier TSs.
