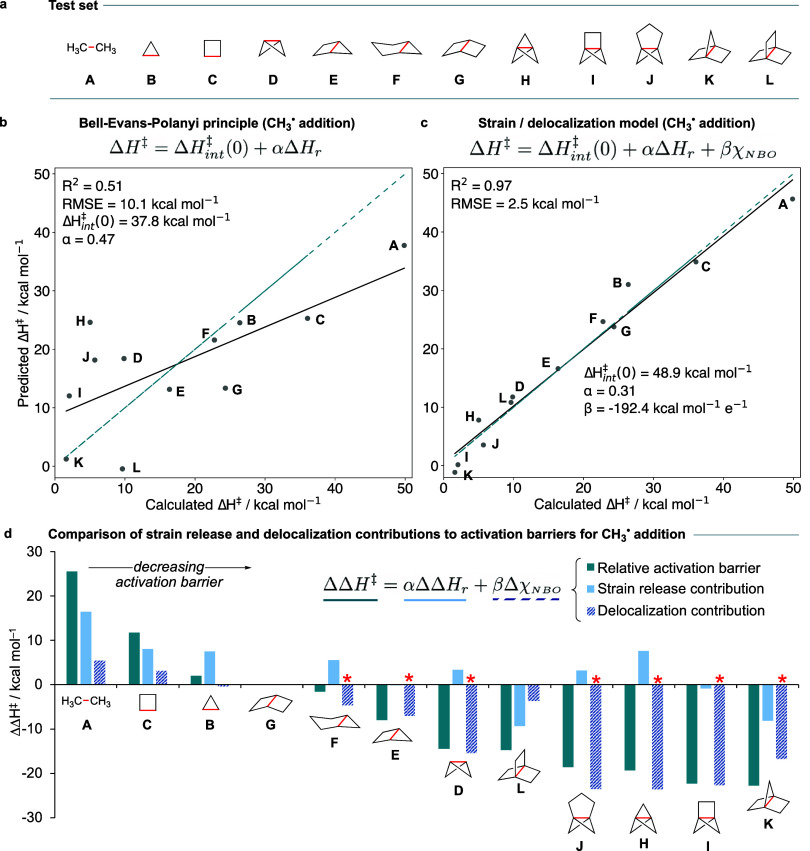
Figure 3.
Delocalization dominates trends in “strain release” ring-opening reactions. (a) Test set of acyclic, monocyclic, and fused polycyclic hydrocarbons. (b) BEP plot (predicted vs calculated ΔH‡, kcal mol–1) for the addition of methyl radical to the red bonds of the molecules in the test set. The blue dashed line denotes perfect correlation. (c) Prediction of ΔH‡ from ΔHr and χNBO (eq 3). (d) Breakdown of strain and delocalization (χNBO) contributions to ΔΔH‡ (kcal mol–1) for the addition of methyl radical to the test set, relative to bicyclo[2.2.0]hexane (G), with α = 0.51 and β = −192.4 kcal mol–1 e–1. Asterisks indicate the cases where delocalization dominates over strain release.