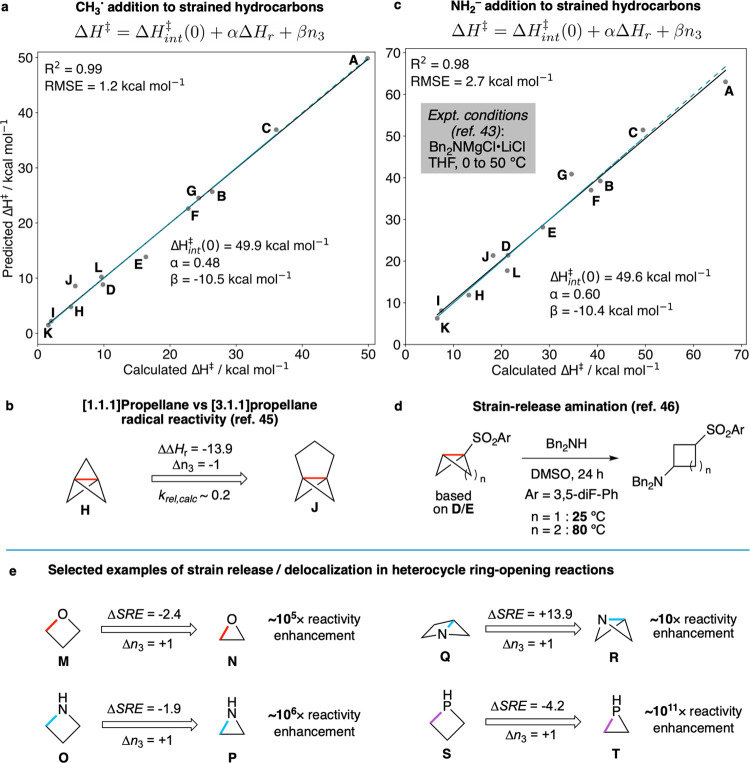
Figure 5.
Implications of strain and delocalization on general reactivity. MLR plots for the prediction of ΔH‡ from ΔHr and n3 for the hydrocarbon test set with CH3• (a) and NH2– (b) using eq 4. The blue dashed lines denote perfect correlation. (c) Increasing strain release driving force for [3.1.1]propellane (J) vs [1.1.1]propellane (H) counteracts the decrease in intrinsic reactivity due to a loss of bond delocalization, resulting in similar reactivity. (d) Addition of dibenzylamine to bicyclo[1.1.0]butane and bicyclo[2.1.0]pentane sulfones. Increased delocalisation lowers the required temperature for this reaction, opposing the expected behavior based on strain release energies alone. . (e) Selected examples of the synergy or antagonism between strain release and delocalization in the ring-opening reactivity of heterocycles. See the SI (Figures S9 and S10) for Marcus Ea values from refs (47) and (48) and the full data set of radical and anionic reactivity. All relative reaction rates were estimated at 298 K.