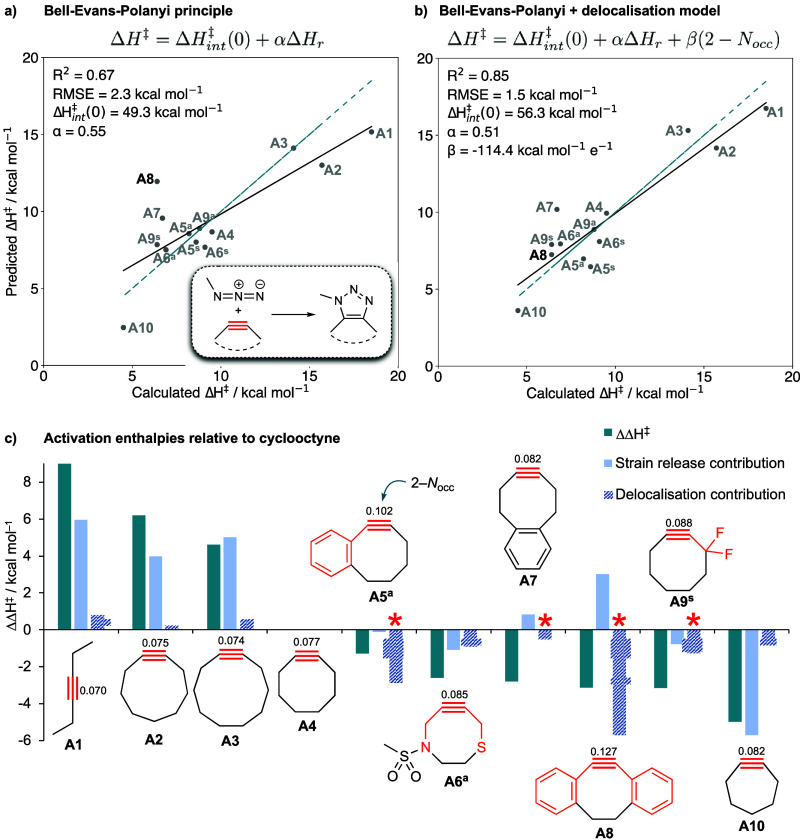
Figure 7.
Application to (3 + 2) azide–alkyne cycloaddition reactions. Delocalization, not strain release, explains the enhanced reactivity of dibenzocyclooctyne over cyclooctyne in (3 + 2) cycloadditions with methyl azide. (a) BEP plot (predicted vs calculated ΔH‡, kcal mol–1) for the addition of methyl azide to the red bonds of the alkynes in the test set. The blue dashed line denotes perfect correlation. (b) Prediction of ΔH‡ from ΔHr and χNBO (eq 3). (c) Breakdown of strain release and delocalization (χNBO) contributions to ΔΔH‡ (kcal mol–1) for the addition of methyl azide to the test set relative to cyclooctyne (A4), following the protocol in Figure 3d. Asterisks indicate the cases where delocalization dominates over strain release, and superscripts a and s refer to anti and syn TSs, respectively.